Scientists at the Wyss Institute for Biologically Inspired Engineering at Harvard University, Harvard Medical School (HMS), and Duke University, in collaboration with Gameto, report that they have created a living, fully human ovarian organoid that supports egg cell maturation, develops follicles, and secretes sex hormones. This “ovaroid” model enables the study of human ovarian biology without the need to take tissue from patients and could enable the development of new treatments for conditions like infertility, ovarian cancer, and more, according to the researchers.
Through an agreement with Harvard’s Office of Technology Development (OTD), the technology has been licensed to Gameto, which is using it to develop therapeutics for diseases of the female reproductive system. The ovaroids are described in detail “Directed differentiation of human iPSCs to functional ovarian granulosa-like cells via transcription factor overexpression” in eLife.
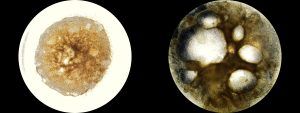
“An in vitro model of human ovarian follicles would greatly benefit the study of female reproduction. Ovarian development requires the combination of germ cells and several types of somatic cells. Among these, granulosa cells play a key role in follicle formation and support for oogenesis. Whereas efficient protocols exist for generating human primordial germ cell-like cells (hPGCLCs) from human induced pluripotent stem cells (hiPSCs), a method of generating granulosa cells has been elusive,” write the investigators.
“Here, we report that simultaneous overexpression of two transcription factors (TFs) can direct the differentiation of hiPSCs to granulosa-like cells. We elucidate the regulatory effects of several granulosa-related TFs and establish that overexpression of NR5A1 and either RUNX1 or RUNX2 is sufficient to generate granulosa-like cells. Our granulosa-like cells have transcriptomes similar to human fetal ovarian cells and recapitulate key ovarian phenotypes including follicle formation and steroidogenesis.
“When aggregated with hPGCLCs, our cells form ovary-like organoids (ovaroids) and support hPGCLC development from the premigratory to the gonadal stage as measured by induction of DAZL expression. This model system will provide unique opportunities for studying human ovarian biology and may enable the development of therapies for female reproductive health.
“Our new method of fully human ovaroid production is several times faster than existing human/mouse hybrid methods, and replicates many of the critical functions of these organs, marking a significant step forward in our ability to study female reproductive health in the lab. In the future, similar technology could also treat infertility by growing egg cells from people whose own eggs aren’t viable,” said co-first author Merrick Pierson Smela, a graduate student in the lab of George Church, PhD, at the Wyss Institute and HMS.
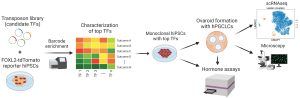
“Creating the granulosa cells on their own was a significant accomplishment, but making an ovaroid out of only granulosa cells wouldn’t tell us anything about their ability to support the maturation of germ cells, which was what we wanted to be able to study in vitro,” said co-first author Christian Kramme, PhD, the vice president of cell engineering at Gameto and a former graduate student in Church’s group at the Wyss Institute and HMS. “This process had been replicated previously using hPGCLCs and mouse somatic cells, but with this new technology, we now have the ability to do it with a fully human model.”
The Wyss team is continuing to develop its human ovaroid model and plans to integrate additional ovarian cell types, including hormone-producing theca cells, to more fully replicate the complex functions of the human ovary. They also hope to improve their culture system to allow their germ cells to fully develop into egg cells, and determine the optimal dosage of the different TFs. Gameto, meanwhile, has conducted preclinical studies of a derived co-culture system for egg maturation in humans with leading national fertility clinics.


