A monoclonal antibody (mAb) against a glycoprotein called repulsive guidance molecule-a (RGMa) may provide an efficient therapeutic strategy for treating neuromyelitis optica (NMO), scientists at Osaka University, Japan, have found in a study conducted on human autopsy samples and a disease-relevant rat model.
NMO is a currently incurable autoimmune condition where the immune system attacks an individual’s own brain, spinal cord, and particularly the optic nerve, causing blindness, paralysis, numbness, neuropathic pain and death.
A key feature of NMO is the inflammation of star-shaped cells called astrocytes around blood vessels in the brain and spinal cord, a condition called “perivascular astrocytopathy.” Astrocytes are critical in several functions including maintaining the blood–brain barrier, providing nutrients to neurons, regulating ion balance, and repairing the brain and spinal cord after an infection or injury.
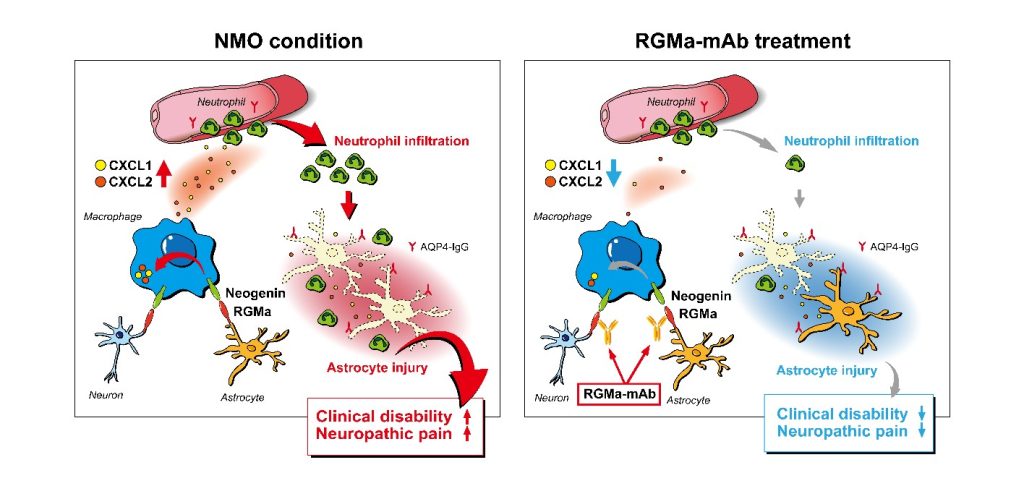
Perivascular astrocytopathy in NMO is mostly caused by the leakage of antibodies against aquaporin-4 (AQP4) into the central nervous system. Anti-AQP4 antibodies leak into the tissue at sites of nerve damage and neutrophils accumulate. This is associated with the death of astrocytes, ultimately resulting in NMO.
“Using a clinically relevant NMO rat model, we evaluated the therapeutic effect of a RGMa-mAb by behavioral testing, immunohistochemistry, and gene expression assay. We further performed in vitro experiments to address the RGMa-signaling in macrophages,” the investigators of the study note.
The new study was published on March 11, 2022, in the journal Annals of Neurology, in an article titled “RGMa signal in macrophages induces neutrophil-related astrocytopathy in NMO.” In this study the researchers provide new insights on the pathophysiological mechanism of NMO and show that interrupting the interplay between immune cells, specifically macrophages and neutrophils, can prevent NMO-linked damage.
To uncover the underlying mechanism, the team used a disease-relevant rat model of NMO to test the effects of anti-RGMa antibody on NMO and associated gene and protein expression profiles.
“Our findings revealed a new molecular mechanism of NMO pathophysiology in which RGMa stimulates macrophages to attract neutrophils to the lesions, where they kill off astrocytes,” said Toshihide Yamashita, PhD, senior author of the study.
Exploiting the new mechanistic insight, the authors treated model rats with a mAb against RGMa which resulted in decreased expression of neutrophil-attracting chemicals, the attraction of fewer neutrophils into the damaged nerve lesions, less astrocytic death, and decreased movement and pain symptoms.
The authors showed, in both NMO rats and an NMO-autopsied human samples, RGMa was expressed by undamaged neurons and astrocytes, whereas neogenin, an RGMa receptor, was present on infiltrating macrophages. The researchers also showed, neogenin-expressing macrophages that accumulate in the nerve lesions expressed CXCL2, a molecule that strongly attracts neutrophils, and RGMa directly regulated CXCL2 expression in macrophages to reduce the infiltration of neutrophils into the damaged nerve lesions.
Based on their preclinical studies reported in this paper, the authors conclude RGMa signaling in infiltrated macrophages drives neutrophil-related astrocytopathy in NMO and mAb against RGMa may provide a treatment for neuropathic pain and movement challenges in patients with NMO.
The study was funded by the Japan Society for the Promotion of Science and the Japan Agency for Medical Research and Development.