
Research in mice has shown how antibiotic use can leave the lungs susceptible to flu virus infections. The studies, headed by scientists at the Francis Crick Institute, found that gut bacteria play an important role in driving interferon (IFN) signaling in non-immune cells in the lung lining, which help to maintain a first line of defense against flu infection. So, while about 80% of mice with healthy gut microbiota survived when infected with flu virus, only about a third of animals survived the flu if they had been pretreated with antibiotics.
“This study supports that taking antibiotics inappropriately not only promotes antibiotic resistance and wipes out the commensals in your gut that are useful and protective, but it may also render you more susceptible to viral infections,” explained Andreas Wack, PhD, group leader who led the research at the Francis Crick Institute. “We found that antibiotics can wipe out early flu resistance, adding further evidence that they should not be taken or prescribed lightly.” The researchers reported their findings in Cell Reports, in a paper titled, “Microbiota-Driven Tonic Interferon Signals in Lung Stromal Cells Protect from Influenza Virus Infection.”
Type 1 interferon (IFNα/β) signaling plays a central role in the immune defense against viral infection, the authors wrote. “More than 60 years of research have established the critical importance to human health of type I IFN (IFNα/β), as it induces transcription of interferon-stimulated genes (ISGs) that encode proteins with various antiviral function.”
However, IFN activity must be finely controlled, as evidence shows that over-production of IFN can cause severe disease. This is seen in individuals with a genetic variant that results in high interferon production. While they can mount enhanced immune responses against viruses, they may also show signs of chronic auto-inflammation. How the body can safeguard the right balance of IFNα/β signaling to maximize antiviral protection while minimizing excessive inflammation isn’t clear. One mechanism for IFNα/β regulation is the transient downregulation of cell surface interferon IFNα/β receptor (IFNAR1) expression although, as the authors noted, “the importance of this host strategy in viral infection has yet to be established.”
To try and answer this question the team studied mice that carry an interferon receptor gene mutation (Ifnar1SA) which means they are unable to downregulate the IFN receptor IFNAR1, and so exhibit enhanced IFN signaling. These animals were found to be more resistant to influenza virus infection than control animals, and exhibited less weight loss, lower virus gene expression eight hours after infection, and reduced influenza virus replication two days later. This early control of the infection meant that it wasn’t necessary to further ramp up IFNα/β signaling and antiviral immune responses because the virus couldn’t get a foothold. “… we found reduced, not increased, IFN protein levels at 48 h post-infection in Ifnar1SA mice, the authors stated. Further testing indicated that “the reduced immune response we find in Ifnar1SA in influenza virus-infected mice is, therefore, a direct consequence of reduced virus load as early as 8 h into the infection.”
Interestingly, the protective effects of enhanced baseline IFNα/β signaling were dampened in animals that were pretreated with antibiotics for 2–4 weeks. Tests showed that antibiotic exposure led to decreased IFNα/β signaling in lung stromal cells, not immune system cells. However, giving the antibiotic-treated mice fecal transplants reversed antibiotic-induced susceptibility to flu virus infection, which suggested a role for gut microbiota in controlling IFN signaling. “To further confirm that gut microbiota are indeed responsible for the observed changes in baseline ISG expression, we treated antibiotic-exposed mice with fecal material of control mice through oral gavage (fecal transplantation [FT]). This treatment reversed the antibiotic-induced ISG reduction in lung stromal cells.”
“We were surprised to discover that the cells lining the lung, rather than immune cells, were responsible for early flu resistance induced by microbiota,” commented Wack. “Previous studies have focused on immune cells, but we found that the lining cells are more important for the crucial early stages of infection. They are the only place that the virus can multiply, so they are the key battleground in the fight against flu. Gut bacteria send a signal that keeps the cells lining the lung prepared, preventing the virus from multiplying so quickly.
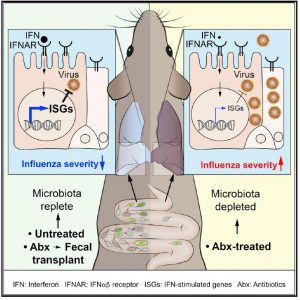
“It takes around two days for immune cells to mount a response, in which time the virus is multiplying in the lung lining. Two days after infection, antibiotic-treated mice had five times more virus in their lungs. To face this bigger threat, the immune response is much stronger and more damaging, leading to more severe symptoms and worse outcomes.”
The findings support previous studies showing that mice treated using oral antibiotics are more susceptible to viral infections, including influenza A. “This and previous studies demonstrate that microbiota-driven signals can act at multiple levels, inducing an antiviral state in non-immune cells to control infection early on, and enhancing the functionality of immune cells later in infection,” noted Wack. “Taken together, our findings show that gut bacteria help to keep non-immune cells elsewhere in the body prepared for attack. They are better protected from flu because antiviral genes are already switched on when the virus arrives. So when the virus infects a prepared organism, it has almost lost before the battle starts. By contrast, without gut bacteria, the antiviral genes won’t come on until the immune response kicks in. This is sometimes too late as the virus has already multiplied many times, so a massive, damaging immune response is inevitable.”
The authors say their results support exercising caution when treating patients with antibiotics. “Between 2000 and 2015, worldwide antibiotic consumption is believed to have increased by 65%, much of which may be linked to inappropriate treatment of pollution-and viral-based illnesses,” they wrote. “Our results suggest that inappropriate use of oral antibiotics could predispose patients to more severe influenza, because of reduced antiviral resistance of the epithelia.” The findings also have implications for agriculture, Wack continued. “This could be relevant not only in humans but also livestock animals, as many farms around the world use antibiotics prophylactically. Further research in these environments is urgently needed to see whether this makes them more susceptible to viral infections.”
The team plans to continue their investigations into the origins and mechanisms underlying microbiota-driven antiviral resistance. “Previous research has suggested that the microbiota-driven signal in lung stromal cells could originate either from the gut or the lung,” Wack noted. “However, in the work presented here, the results of the fecal transplant experiments strongly suggest a gut involvement in this effect. We would love to understand the exact nature of the signal from the gut to the lung, and we are working on several hypotheses.”





