Researchers have developed an injectable inflammation-responsive hydrogel (IRH), which tests in mice showed can deliver an anti-inflammatory drug directly to the retina of the eye. Reporting on in vitro experiments and then on tests in mice with retinal degeneration—a model of retinitis pigmentosa (RP), the team found that the hyaluronic acid (HA)-based hydrogel, loaded could successfully deliver an anti-inflammatory EZH2-inhibitor drug to the retina in response to intraocular inflammation.
Their results showed that treatment not only reduced intraretinal inflammation in the treated animals’ eyes, but also protected the photoreceptors morphologically and functionally. The team suggests the IRH could be developed into a treatment to hold back the damage resulting from diseases such as retinitis pigmentosa. Maesoon Im, PhD, at the Korea Institute of Science and Technology Brain Science Institute, along with colleagues Seung Ja Oh, PhD, at Kyung Hee University and Kangwon Lee, PhD, at Seoul National University, reported on their development in npj Regenerative Medicine.
“For future commercialization, we plan to digitize the amount of drug and hydrogel used, as well as the treatment period, according to the progression of the disease,” said Maesoon Im. “We also intend to assess the long-term stability of the drug delivery system.” Added Seung Ja O, “In addition to the retinal degenerative diseases, we will investigate inflammation levels in other retinal diseases to see if our inflammation-responsive drug delivery system would work on those conditions.”
In their paper, titled “Effective protection of photoreceptors using an inflammation-responsive hydrogel to attenuate outer retinal degeneration,” the team commented, “Our results suggest the IRH reported here can be used to considerably delay vision loss caused by RP.”
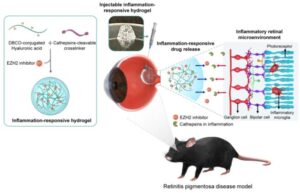
There is no effective cure for either RP or AMD. One current form of treatment involves an injection of anti-inflammatory drugs into the eye to slow down the degree of retinal damage. However, these injections only work for as long as the drug remains in the eye, requiring patients to visit a clinic for intraocular injections every four to 12 weeks, depending on how long the effect of the drug lasts. “Although effective regulation of intraretinal inflammation can slow down the progression of the disease, an efficient anti-inflammatory treatment strategy is still lacking,” the authors stated. And while some drawbacks of current treatments could be addressed by the use of an appropriate drug carrier for intraocular administration, “… its development has been challenging because eye is a highly complexed and specialized organ of the body,” they pointed out.
Im and colleagues have now generated a potential treatment that utilizes a small molecule inhibitor of an inflammatory factor EZH2—which studies suggest contributes to retinal degeneration—formulated into a hyaluronic acid-based hydrogel. “… it has been reported that enhancer of zeste homolog 2 (EZH2) is overexpressed in the degenerate retina of a mouse model, contributing to rod cell death,” the investigators pointed out. “Also, the inhibition of EZH2 effectively attenuated level of inflammatory microglia in a neuroinflammatory disease.”
Given previous research demonstrating that EZH2 contributes to rod photoreceptor death and and microglial inflammation, the team reasoned that “selective modulation of the EZH2 in a controlled manner could be a favorable approach for safe and effective therapeutic outcomes in retinal degenerative diseases which are associated with inflammation activities.” However, they acknowledged that EZH2 suppression had not been studied as a therapeutic approach for any retinal degenerative diseases.
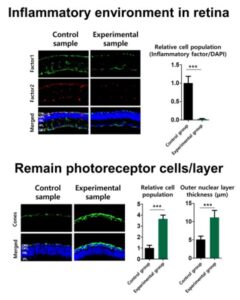
For their newly reported study the team developed a hydrogel that would slowly degrade upon encountering the enzyme cathepsin, which is typically overexpressed in inflammatory environments, and deliver the anti-inflammatory EZH2 inhibitor. Their in vivo tests in a mouse model of RP confirmed that retinal degeneration slowed in animals receiving injections of the EZH2 inhibitor-loaded IRH. In the recipient animals inflammatory factors in the retina were reduced to approximately 6.1%. The findings also showed that treatment resulted in a protective effect on photoreceptor cells, effectively delaying vision loss. “Collectively, our results indicate that treatment using an IRH loaded with an EZH2 inhibitor can effectively slow down the degradation of physiological functions of the retina, in addition to the reduced inflammation activities, both which arise from RP,” they stated.
The investigators envisage that their newly developed technology could reduce the economic burden and the risk of accidents during outpatient visits for patients with difficulty in mobility due to visual impairment. Additionally, for patients who are experience early stages of symptoms, reducing the frequency of hospital visits could alleviate inconvenience in daily life.
Summarizing their work, the team stated, “… our results indicate that treatment using an IRH loaded with an EZH2 inhibitor can effectively slow down the degradation of physiological functions of the retina, in addition to the reduced inflammation activities, both which arise from RP … Collectively, on-demand EZH2 inhibitor delivery using the IRH may effectively alleviate vision loss caused by RD.” Acknowledging the need for further development, the team also considered the wider potential applications of the technology. “The hydrogel platform developed in this study can be applied to diverse inflammatory diseases that require minimally invasive treatment in combination with various drugs. For clinical applications, further study is necessary to develop inflammation-responsive hydrogels that actively control disease and enable long-term drug release.”


