A research team says it has shown for the first time that human brain organoids implanted in mice have established functional connectivity to the animals’ cortex and responded to external sensory stimuli. The implanted organoids reacted to visual stimuli in the same way as surrounding tissues, an observation that researchers were able to make in real time over several months thanks to an experimental setup that combines transparent graphene microelectrode arrays and two-photon imaging.
The scientists published their study “Multimodal monitoring of human cortical organoids implanted in mice reveal functional connection with visual cortex” in Nature Communications.
Human cortical organoids are derived from human induced pluripotent stem cells, which are usually derived themselves from skin cells. These brain organoids have recently emerged as promising models to study the development of the human brain, as well as a range of neurological conditions.

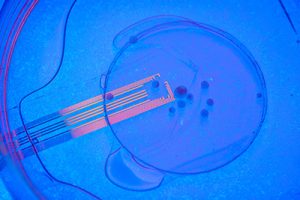
The UC San Diego-led team was able to solve this problem by developing experiments that combine microelectrode arrays made from transparent graphene, and two-photon imaging, a microscopy technique that can image living tissue up to one millimeter in thickness.
“Human cortical organoids, three-dimensional neuronal cultures, are emerging as powerful tools to study brain development and dysfunction. However, whether organoids can functionally connect to a sensory network in vivo has yet to be demonstrated,” write the investigators.
“Here, we combine transparent microelectrode arrays and two-photon imaging for longitudinal, multimodal monitoring of human cortical organoids transplanted into the retrosplenial cortex of adult mice. Two-photon imaging shows vascularization of the transplanted organoid. Visual stimuli evoke electrophysiological responses in the organoid, matching the responses from the surrounding cortex. Increases in multi-unit activity (MUA) and gamma power and phase locking of stimulus-evoked MUA with slow oscillations indicate functional integration between the organoid and the host brain.
“Immunostaining confirms the presence of human-mouse synapses. Implantation of transparent microelectrodes with organoids serves as a versatile in vivo platform for comprehensive evaluation of the development, maturation, and functional integration of human neuronal networks within the mouse brain.”
Simultaneously recorded optically and electrically for the first time
“No other study has been able to record optically and electrically at the same time,” said Madison Wilson, the paper’s first author and a Ph.D. student in the lab of Duygu Kuzum, PhD, a faculty member in the University of California San Diego department of electrical and computer engineering. “Our experiments reveal that visual stimuli evoke electrophysiological responses in the organoids, matching the responses from the surrounding cortex.”
The researchers hope that this combination of innovative neural recording technologies to study organoids will serve as a unique platform to comprehensively evaluate organoids as models for brain development and disease and investigate their use as neural prosthetics to restore function to lost, degenerated or damaged brain regions.
Next steps include longer experiments involving neurological disease models, as well as incorporating calcium imaging in the experimental set up to visualize spiking activity in organoid neurons. Other methods could also be used to trace axonal projections between organoid and mouse cortex.
“We envision that, further along the road, this combination of stem cells and neurorecording technologies will be used for modeling disease under physiological conditions; examining candidate treatments on patient-specific organoids; and evaluating organoids’ potential to restore specific lost, degenerated or damaged brain regions,” Kuzum noted.







