The early CRISPR-Cas9 gene editing systems didn’t lack for brashness. They quickly set new standards for economy and ease of use. They even seemed to offer high degrees of accuracy and efficiency. But soon, brashness started to show its limits. What was needed in its place was refinement.
Possible alternatives to CRISPR-Cas9 included a transposase system that predated CRISPR-Cas9, as well as a newer dimeric system that incorporated a pair of deactivated Cas9 (dCas) nucleases along with several other elements. The first system, piggyBac, and the second system, Cas-CLOVER, underwent early development at Transposagen Biopharmaceuticals, which launched a trio of sister companies—Hera BioLabs, Poseida Therapeutics, and Demeetra AgBio—to further the development of piggyBac and Cas-CLOVER for different applications. (Incidentally, Transposagen is now known as “a Hera BioLabs company.”)
Hera BioLabs was launched in 2014 and focuses on services in drug discovery and preclinical research. Poseida Therapeutics was also launched in 2014, and it focuses on cellular immunotherapies and gene therapies. Finally, Demeetra AgBio, which was launched in 2019, focuses on applications in industrial biotechnology and agriculture. This article focuses on Demeetra AgBio, which declares that its mission is to “optimize food production, enhance synthetic biotechnology, and open up a new world of therapeutics.”
Doubling up … then bearing down
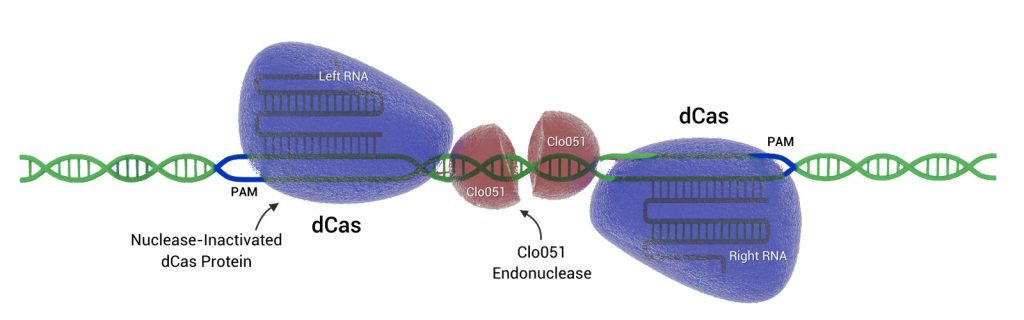
In CRISPR-Cas9 systems, the brashest bit is Cas9, an endonuclease that can target DNA sequences using little more than a single guide RNA (gRNA). Cas-CLOVER is not so easily loosed upon the genome. Cas-CLOVER utilizes two gRNAs and two fusion proteins, where each fusion protein consists of a deactivated Cas9 (dCas9) nuclease and a subunit of an obligate dimer nuclease called Clo051.
Because the activity of Clo051 is contingent upon its dimerization, DNA cleavage by Cas-CLOVER is strictly dependent on the simultaneous on-target binding of the two distinct gRNA-guided endonucleases. Moreover, the when the endonucleases bind, they must be within a specific distance of each other.

CEO, Demeetra AgBio
“Even if one of the paired halves binds off-target, the nuclease will not cut or nick the genome,” says Jack Crawford, Demeetra AgBio’s CEO. “The paired halves have to come together at the intended site for cutting to occur.”
Another advantage of Cas-CLOVER’s dual-gRNA targeting system is that it can be used to obtain large deletions. Indeed, deletions of around 8–50 base pairs may be obtained, whereas CRISPR-Cas9 typically results in deletions of just 1–3 base pairs.
When deletions are larger, knockout edits are more likely to be full and clean. Also, large deletions can facilitate the identification of edited cells. Simple gel-based screening methods may suffice.
Cultivating diversity
Cas-CLOVER works in multiple cell types. “We validated it plant cells, CHO cells, yeast cells, and HEK293 cells, which are important for viral vector biomanufacturing,” Crawford elaborates. “Cas-CLOVER can be used to engineer cell lines, yeast, algae, animals, or plants so that they express desired proteins and traits.”
Crawford remarks that the biopharma industry can access Cas-CLOVER through a “simple, single-source commercial license from Demeetra AgBio.” Licensing Cas-CLOVER in the bioprocessing field usually begins with contacting Demeetra AgBio to order reagents.
“Customers [then] have a period of time—we start with six months—to test the technology internally,” Crawford notes. “If they like it, they can convert to a commercial license.” Other options, such as a one-time fee, royalties, or milestone-based agreements, also are available to fit the size and needs of partners.
For example, one of Demeetra AgBio’s partners wanted to increase its ability to select for high-yielding clones in CHO cells. “It had at least 30 different cell lines, each of which might have different characteristics,” Crawford details. “Using Cas-CLOVER, its team made glutamine synthetase knockouts in a matter of weeks. Consequently, it now has a reliable selection system for high-yielding clones.” Other examples include partners that are using Cas-CLOVER to enhance glycosylation or antibody-dependent cellular cytotoxicity mechanisms.
Flexible deliveries, scarless removals
In addition to Cas-CLOVER, Demeetra AgBio works with a proprietary piggyBac gene editing technology. Like Cas-CLOVER, this piggyBac technology is shared with Hera BioLabs and Poseida Therapeutics.
The piggyBac DNA Delivery System is a transposon-based system that can introduce potentially very large genetic cargoes, such as therapeutic transgenes, to the genome. More specifically, for synthetic biotechnology in cell lines, yeast, and other microorganisms, piggyBac preferentially integrates genes of interest into highly expressed sites in the genome.
“piggyBac is the workhorse that gets large DNA cargo into the genome,” says Eric Ostertag, MD, PhD, executive chairman of Poseida Therapeutics and chairman of the board of Demeetra AgBio. Originally, piggyBac was used to engineer induced pluripotent stem cells, and now it is used with many cell types and organisms. Newer iterations, called Super piggyBac, contain “hyperactive” mutations that greatly increase the activity of the enzyme.
Looking back, looking ahead
“I founded Transposagen Biopharmaceuticals about 20 years ago as a pioneering gene therapy company,” Ostertag states. He had worked with viruses and a retrotransposon as the first graduate of the University of Pennsylvania’s gene therapy program. This experience convinced him that gene therapy would never be fully successful if it relied on adeno-associated viruses or other viral-based technologies for gene delivery.
“To solve some of the problems of viruses, we started stripping out some of the viral components,” Ostertag recalls. “But I realized that approach wouldn’t be able to solve all the problems, and that it could actually create new ones, such as losing the ability to stably integrate into the genome.
“Therefore, I started working on a completely nonviral solution using nanoparticles. However, we still needed a safe way to integrate potentially large pieces of DNA into the genome. The solution was piggyBac.”
During that time, site-specific genome editing technologies were evolving. For example, systems based on zinc finger nucleases, transcription activator-like effector nucleases, and then CRISPR nucleases were introduced. “We immediately recognized the value of CRISPR-Cas9, as well as its disadvantages,” Ostertag says. “And we began working on alternatives that would eliminate the disadvantages.”
The alternatives developed by Ostertag and his colleagues include the Cas-CLOVER and piggyBac systems. Today, these systems are being advanced in different contexts by Hera BioLabs, Poseida Therapeutics, and Demeetra AgBio.
A key challenge, says Demeetra AgBio’s Crawford, is “getting the message out about Cas-CLOVER and validating this system for other applications, such as afucosylated antibodies and other antibodies used in bioprocessing.”
“We can license the Cas-CLOVER system in our fields of use,” Crawford says, “but we also offer reagents and cell lines in those same fields of use.” Demeetra AgBio is also building an in-house program to develop analogs to cannabinoid therapeutics. He relates, “We’re in preclinical testing for our lead compounds.”
Beyond cannabinoids, the company is interested in using afucosylated CHO cells to make biosimilars and what it calls bio-betters. For example, it can use Cas-CLOVER to knock out certain genes to change the glycosylation of antibodies, which can then be enhanced.
Crawford expects to introduce new, more efficient variants of Cas-CLOVER around the end of the year. “In a head-to-head comparison, they’re much better than CRISPR-Cas9,” he asserts.