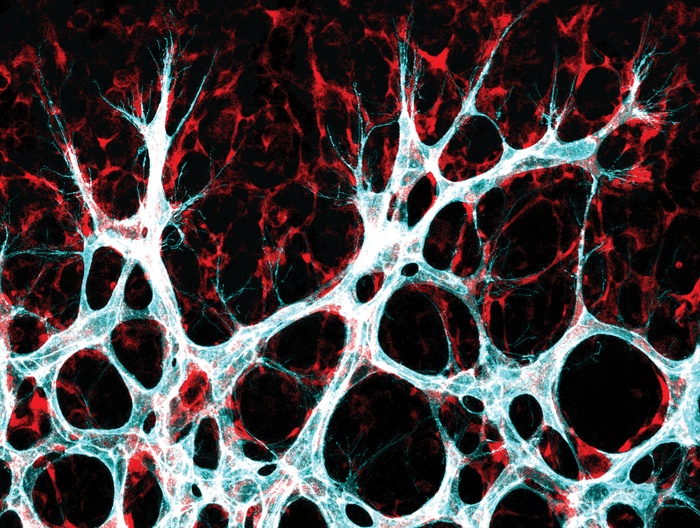
Blood vessels run throughout the human body and ensure that our organs get all the nutrients and oxygen they need. An international research group has now discovered how two proteins named YAP and TAZ play a crucial role in allowing new blood vessels to sprout, even under challenging metabolic conditions. The proteins are part of the Hippo signaling pathway, which regulates organ growth and size in almost all living things.
Michael Potente, PhD, and Holger Gerhardt, PhD, and their research teams now want to study how much the mechanism—which they described during tissue development—is also involved in regeneration and repair processes that rely heavily on blood vessels. “We’re primarily interested in finding out whether and, if relevant, how malfunctions in that signaling pathway can cause vascular diseases in humans,” said Potente, who is a professor for translational vascular biomedicine at the Berlin Institute of Health at Charité (BIH) and a guest researcher at the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC). Gerhardt is head of the Integrative Vascular Biology Laboratory at the MDC, and works next door to Potente in the Käthe Beutler Building in Berlin-Buch.
The researchers reported on their work in Nature Metabolism, in a paper titled, “A YAP/TAZ-TEAD signaling module links endothelial nutrient acquisition to angiogenic growth.”
Blood vessels form extensive tubular networks of arteries, capillaries, and veins that nurture all body tissues, the authors wrote. If these finely woven networks stop working as they should, we risk developing diseases.
While age-related cardiovascular conditions frequently cause blood vessels to atrophy, malignant tumors are characterized by excessive growth of misrouted vessels. Wet macular degeneration is also associated with the sprouting of new blood vessels in the wrong place. At its worst, this condition can cause blindness.
Endothelial cells (ECs) line the inner surface of blood vessels, where they are surrounded by diverse nutrients such as amino acids, glucose, and lipids.” Angiogenesis, the process by which endothelial cells form new blood vessels from existing ones, requires increased nutrient uptake and consumption by these cells to meet the increased metabolic demands. The authors further pointed out, “Controlling nutrient acquisition and usage is, therefore, central to the function of the endothelium; yet, the mechanisms that regulate these processes are poorly understood … how endothelial nutrient acquisition and usage are regulated is unknown.”
Potente said, “To help us develop targeted therapies for these kinds of disease, we want to find out how exactly the growth of new blood vessels—a process called angiogenesis—is regulated within the body. His angiogenesis & metabolism laboratory is part of the Berlin Center for Translational Vascular Biomedicine, an interdisciplinary facility that is a joint focus area of the BIH, Charité—Universitätsmedizin Berlin, and the MDC.
The findings are based on mouse experiments. The mouse retina is an ideal model for studying blood vessel development. “YAP and TAZ are effectors of the Hippo pathway and essential regulators of vascular development, whose activity is highly sensitive to changes in the micro-environment,” the authors said. “By sensing and responding to mechanical, metabolic, and soluble signals, these proteins coordinate tissue growth responses.”
“Using genetically modified mouse lines, we showed how endothelial cells that don’t produce YAP and TAZ almost never divide,” said Potente. “This inhibited vessel growth in the mice.” The TAZ protein plays an especially important role in this process, while YAP is the decisive factor in most other types of cells. Compared to YAP, TAZ was also the more abundant transcript in various endothelial subtypes suggesting a critical role of TAZ for YAP/TAZ responses in the endothelium,” the team noted. They further explained, “ECs lacking YAP/TAZ or their transcriptional partners, TEAD1, 2, and 4 fail to divide, resulting in stunted vascular growth in mice. Conversely, activation of TAZ, the more abundant paralogue in ECs, boosts proliferation, leading to vascular hyperplasia.”
The new findings have shown that if YAP and TAZ are active in the cells of the endothelium, they effectively read genes that lead to increased growth of certain surface transporters, Potente said. “These allow the vessel cells to absorb more nutrients that are important for growth and cell division.” YAP and TAZ, which both function in a similar way, therefore act as a kind of door-opener.
“Because new blood vessels frequently form in tissues with a poor blood supply, endothelial cells must be able to grow in the most challenging metabolic conditions,” said Potente. “That’s why it’s so important for these cells to have molecular machinery that recognizes and reacts to subtle changes in the extracellular milieu.”
He further explained, “This increased absorption of nutrients leads to the activation of another protein, called mTOR.” mTOR is an important control point in the cells that trigger growth and cell division. “This allows new blood vessel networks to expand.”
The team does not yet know which signals regulate the activity of YAP and TAZ in endothelial cells. Nevertheless, Gerhardt noted, “Together, we’ve discovered a mechanism that enables blood vessels to align their growth closely to the situation in their surroundings. The mechanism stops endothelial cells from dividing if the metabolic resources needed for the process aren’t there.”
The authors concluded, “Our study identifies an essential metabolic link between YAP/TAZ and angiogenic growth, which involves the master regulator of cellular anabolism, TORC1 … Delineating the mechanisms that determine how ECs take up, transport, and use nutrients will provide new insights not only into vascular (patho-)physiology but also into the role of specific nutrients in instructing vascular growth and function.”