Although cancer drug pipelines have produced the occasional gusher in recent years, there have been even more blockages. Indeed, many of the blockages suggest that existing small-molecule and biologic modalities may be unsuitable for certain cancers. In any case, conventional methods for drugging oncogenes and screening compounds have been showing their limitations. Consequently, new methods are being explored.
Biotechnology companies are constructing new discovery platforms and developing completely novel types of drugs. Some of the companies are convinced that innovative methods will show that supposedly undruggable targets are, in fact, druggable. And other companies are interested in showing that targets that have already been “drugged” can be, well, “better drugged.”
Leveraging mRNA
Strand Therapeutics is attacking cancer through the use of mRNA therapeutics combined with synthetic biology principles. The company’s platform is focused around addressing three problems with first-generation mRNA therapeutics, says Jake Becraft, PhD, Strand’s CEO and co-founder. The first problem is rapid degradation. Messenger RNA lasts only a day or two in cells. Strand is working with longer-lasting types of mRNA. These include self-replicating mRNAs that not only extend the lifetime of the molecule, but also increase the level of expression.
The second problem is lack of specificity. According to Becraft, elevated levels of expression require the ability to control expression. “We design different sorts of genetic circuits which allow us to control where in the body or in which types of tissues the mRNA will be active,” he says. He adds that the genetic circuits also allow the mRNA to be inactive in nontarget tissues.
Strand is addressing the third problem—toxicity—through computational biology techniques and innovative manufacturing approaches. “We’re able to make ultralow immunogenicity molecules of mRNA and actually control how they interact with the different immunological sensors within the body,” Becraft explains. “[Doing so] allows us to drop the immunogenicity down orders of magnitude lower than similar vectors.”
Target-wise, Strand is currently working with well-validated targets, with the potential later to build out its pipeline with targets discovered in-house. For its first drug, Strand is targeting tumors with mRNAs encoded with cytokines that express at a high level inside the cell, leading to a systemic immune reaction against the tumor.
Cytokines have been used against tumors before, but they have been delivered from outside the cell. Becraft says this is one of the first approaches using the cell machinery to produce cytokines inside the tumor cell from an mRNA therapeutic. The idea is to achieve a higher concentration and duration of exposure to the cytokines using the localized mRNA-mediated expression.
Strand expects that its lead therapeutic candidate, an intratumorally administered mRNA packaged in a lipid nanoparticle, will enter the clinic in 2023. In a mouse model, treatment with this drug led to what Becraft calls “massive” suppression of tumor growth, with 60–80% of mice showing complete tumor eradication. In a follow-up study in which the drug was administered in combination with an anti-PD1 agent, 100% of mice were cured.
The so-called undruggable target
Some of the most attractive targets in the field of oncology are classified as “undruggable” due to a lack of potential binding pockets. Notable examples include RAS and MYC.
To discover compounds that can bind to such targets, Perturba Therapeutics is using live-cell-based platforms for mammalian membrane two-hybrid (MaMTH) and split-intein-mediated protein ligation (SIMPL) assays. These platforms leverage artificial intelligence (AI)-augmented discovery technology from Cyclica, Perturba’s parent company.

Perturba is using the assays to learn how to drug formerly undruggable targets, including protein-protein interactions (PPIs), and to shorten the discovery time from an average of three to five years to several months. “With these two live-cell assays,” says Igor Stagljar, PhD, chief science officer at Perturba, “we can take any druggable PPI and run it through Cyclica’s AI platform, identify compounds, and then see whether these compounds are inhibiting PPIs or not.”
According to Stagljar, the assays offer a speed advantage. MaMTH and SIMPL select for permeability and toxicity of the compounds, avoiding a time- and labor-intensive follow-up procedure.
Cyclica’s machine learning engines marry knowledge- and structure-based approaches to generate high-quality drug-target interaction (DTI) predictions. For example, Cyclica’s MatchMaker engine takes a panoramic view of the proteome and can systematically map DTIs to observed or inferred ligand-binding sites from experimentally determined protein structures and homology models. In addition, MatchMaker considers the polypharmacology of small molecules, looking at dozens to hundreds of off-target effects that could be exploited to optimize therapies for selectivity and off-target toxicity.
MatchMaker provides the largest, fastest, and most predictive proteome-wide screening capability in the market, asserts Naheed Kurji, co-founder, president, and CEO of Cyclica and president and CEO of Perturba. “Perturba combines Cyclica’s AI drug design platform and drug discovery expertise with experimental live-cell phenotypic assays from the Stagljar laboratory at the University of Toronto,” Kurji adds. “[This approach] represents a paradigm shift in drugging targets that have been recalcitrant to other approaches, and it offers unparalleled speed.”
Perturba is advancing two programs for osimertinib-resistant triple-mutant EGFR non-small cell lung cancer, and recently kicked off multiple programs targeting small GTPases mutated in pancreatic, colon, and non-small cell lung cancer.
Antibody-guided protein degraders
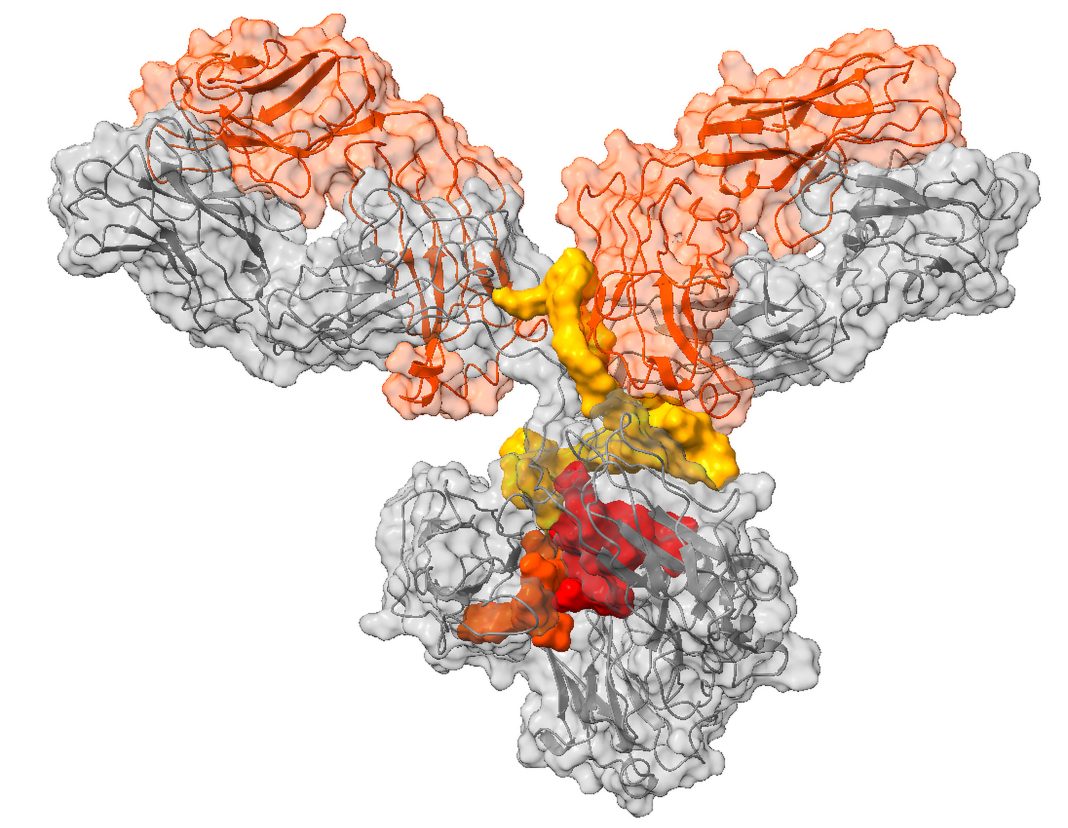
Orum Therapeutics develops tumor-directed targeted protein degraders (TPDs) to treat cancer. These TPDs reflect the company’s TPD2 approach, which addresses undruggable targets by combining the power of protein degradation and the precise tumor cell delivery mechanism of an antibody. The TPD2 approach is realized in several Orum platforms, including the Antibody neoDegrader Conjugate (AnDC) platform, which generates first-in-class antibody-drug conjugates.
According to Orum, the AnDC platform offers an advantage over existing TPD platforms in that the delivery of protein degrader drug conjugates is limited to the target cell. Once in the cell, the mechanism of action is catalytic and can be used many times within the cell.
Tumor-directed TPDs need to outperform conventional small-molecule TPDs with respect to efficacy pharmacokinetics, and safety, says Sung Joo Lee, PhD, CEO of Orum. He relates that the inspiration for Orum’s platform originated from the observation that protein degraders have the potential to address many undruggable targets.
Although Orum’s scientists were intrigued by protein degraders, they recognized that existing approaches would face significant toxicity issues. For example, Lee was aware that a Phase I trial of protein degrader CC-90009 (Celgene/Bristol Myers Squibb) in acute myeloid leukemia and myelodysplastic syndrome had been suspended due to an “adverse change in the risk/benefit.”
That insight led Orum to invest in antibody-drug conjugate tools for TPD delivery. “It was a steep learning curve,” Lee recalls. “The concept is simple, but technically there are a lot of things that need to be considered.” That process involved testing multiple methods of conjugation with multiple versions of the degrader on multiple types of antibodies to determine the best combination.
Orum settled on GSPT1, a translation termination factor, as its first target. When GSPT1 is degraded, translational termination is disrupted and stress on the cell increases, leading to apoptosis. The company’s GSPT1 degrader, ORM-6151, is intended to treat CD33-positive acute myeloid leukemia. Orum plans to file an IND application for ORM-6151 next year.
In solid tumors, Orum is developing a HER2/HER3-targeted protein degrader (ORM-5029) using pertuzumab as the linked antibody. In HER2-expressing cell lines, ORM-5029 showed 10- to 100-fold superior potency compared to HER2 ADCs or GSPT1 degraders.
The virus-like drug conjugate
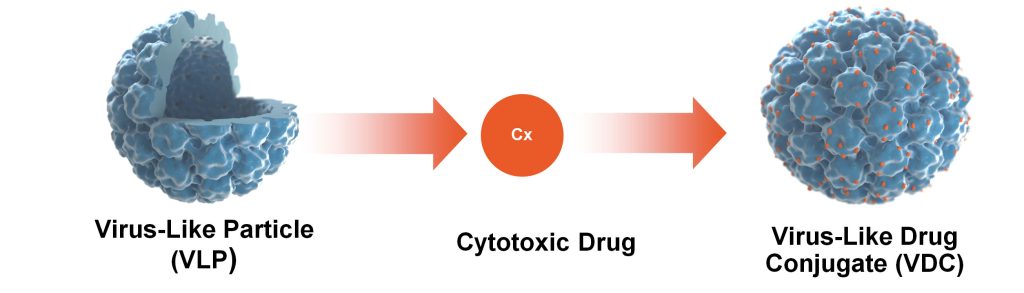
Another company pioneering its own class of drugs is Aura Biosciences. Aura is developing novel biologic drugs called virus-like drug conjugates (VDCs) made of virus-like particles (VLPs) derived from human papillomavirus, linked with cytotoxic drug compounds. In this case, the VLP acts as the targeting system, as the antibody does in an antibody-drug conjugate (ADC), while also providing a secondary mechanism of action by stimulating an immune response.
According to Aura’s CEO, Elisabet de los Pinos, PhD, the inspiration for the company’s platform was the relationship between viruses and cancers. She recalls, “I thought that we should use the affinity and avidity of viruses to cancer cells to basically deliver weapons against them.”
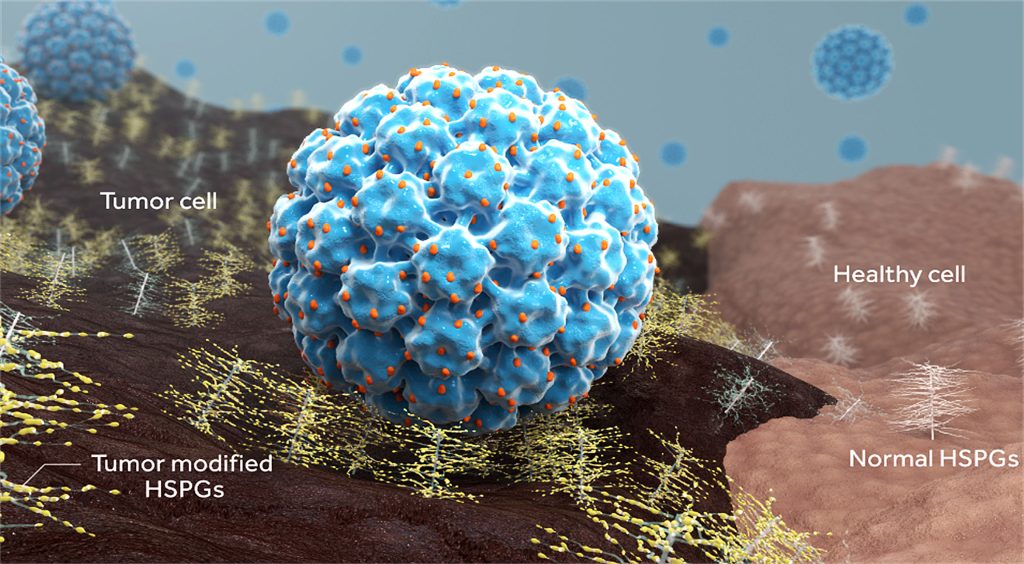
Back in 2015, when Aura was founded, other companies were trying to develop the first viral therapies based on oncolytic viruses. Such therapies, however, rely on viral replication to infect cancer cells, require intratumoral administration, and lead to viral shedding. In contrast, VLPs are very safe as they are made only of the capsid of the virus and cannot replicate. The key innovation was to incorporate the tools of ADCs to create a VDC.
“One of the advantages of this type of drug is that in an ADC you can usually conjugate five to seven cytotoxic drugs,” de los Pinos remarks. “With a VDC, we’ve been able to conjugate up to 400 molecules.” She adds that VLPs bind the cancer cell in a unique way. Typically, ADCs target receptors such as the epidermal growth factor receptor. But VLPs bind to heparan sulfate proteoglycans, a surface protein commonly overexpressed on cancer cells.
Aura’s lead product is a VDC for early-stage primary choroidal melanoma, a cancer of the eye. In the early stages of disease, the tumor is confined to the eye, and the standard-of-care treatment, radiotherapy, typically leaves the patient blind. “We have the clinical data now to show that it works,” de los Pinos asserts. “It preserves vision.” She adds that if it is administered early, it could, given its mechanism of action, “prevent patients from developing metastatic disease.”
Aura is also planning a Phase I trial in non-muscle-invasive bladder cancer. This is another indication where a treatment exists, but leaves the patient with significant morbidity, with the loss of the bladder.
De los Pinos declares that she is optimistic about the platform potential for VDCs. “We can deliver nucleic acids,” she explains, “and we’re going to be looking at the platform and the growth of the platform broadly, not limited by a particular cytotoxic payload.”