Would you wear clothing or, say, shoelaces or a belt made of muscle fibers? What if those fibers could endure more energy before breaking than cotton, silk, nylon, or even Kevlar, and be produced without harm coming to any animals?
Researchers at the McKelvey School of Engineering at Washington University in St. Louis have now developed a synthetic chemistry technology that could make this feasible. The method allows them to polymerize proteins inside of engineered microbes. Using the technology, the team engineered microbial production of the high molecular weight muscle protein, titin, which was then spun into fibers. Tests showed the fibers outperformed many synthetic and natural polymers.
“The beauty of the system is that it’s really a platform that can be applied anywhere,” said Cameron Sargent, a PhD student in the division of biological and biomedical sciences. “We can take proteins from different natural contexts, then put them into this platform for polymerization and create larger, longer proteins for various material applications with a greater sustainability.” Sargent, and Christopher Bowen, PhD, who was of the department of energy, environmental & chemical engineering, and is now a senior scientist at Pfizer, are first authors of the team’s paper in Nature Communications, which is titled, “Microbial production of megadalton titin yields fibers with advantageous mechanical properties,” in which they concluded, “These fibers have potential applications in areas from biomedicine to textiles, and the developed approach, coupled with the structure-function insights, promises to accelerate further innovation in microbial production of high-performance materials.”
Biology is a great source of inspiration for materials design, as nature can produce many high-performance, biodegradable materials efficiently, from renewable resources, and using only low energy processes, the authors noted. Exceptionally tough insect silks, and the underwater adhesive byssus produced by mussels are just a couple of examples. And in many cases, the researchers pointed out, “… these natural materials can outperform the best available petroleum-based alternatives.”
It’s not always possible to harvest such materials from their native sources, and scientists can’t always generate synthetic methods that mimic the natural biosynthetic processes at scale. “… engineered microbial production strategies are needed to facilitate the practical use and development of these high-performance, renewable materials,” the team continued.
The synthetic muscle protein produced in the lab of Fuzhong Zhang, PhD, professor in the department of energy, environmental & chemical engineering, has now produced the synthetic muscle protein, titin, which is one of the three major protein components of muscle tissue. Critical to the mechanical properties of titin is its large molecular size. “It’s the largest known protein in nature,” said Sargent. Muscle fibers have been of interest for a long time, Zhang said. Researchers have been trying to design materials with similar properties to muscles for various applications, such as in soft robotics. “We wondered, ‘Why don’t we just directly make synthetic muscles?’” he said. “But we’re not going to harvest them from animals, we’ll use microbes to do it.”
Engineered microbes can be used for scalable production of some small-molecule compounds, but the direct microbial production of polymers with high mechanical performance is limited, as many high-performance natural materials are based on ultra-high molecular weight (UHMW) proteins with highly repetitive amino acid sequences. These UHMW repetitive proteins are, the scientists noted, “extremely difficult to produce in microbes due to genetic instability, low translation efficiency, and metabolic burden.”
To circumvent some of the issues that typically prevent bacteria from producing large proteins, the research team engineered bacteria to piece together smaller segments of the titin protein into UHMW polymers of around two megadaltons in size, which is about 50 times the size of an average bacterial protein. They then used a wet-spinning process to convert the proteins into fibers that were around ten microns in diameter, or a tenth the thickness of human hair.
Working with collaborators Young Shin Jun, PhD, professor in the department of energy, environmental & chemical engineering, and Sinan Keten, PhD, professor in the department of mechanical engineering at Northwestern University, the group then analyzed the structure of these fibers to identify the molecular mechanisms that enable their unique combination of exceptional toughness, strength, and damping capacity, or the ability to dissipate mechanical energy as heat. Structural analyses suggested that these UHMW titin fibers contain axially aligned, side-by-side pairs of Ig-like domains. “Structural analyses and molecular modeling suggest these properties derive from unique inter-chain crystallization of folded immunoglobulin-like domains that resists interchain slippage while permitting intra-chain unfolding,” the researchers wrote.
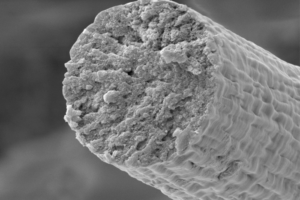
They say that to their knowledge, the achievement represents the first example of an engineered macroscale material produced from titin. “By harnessing the biosynthetic power of microbes, this work has produced a novel high-performance material that recaptures not only the most desirable mechanical properties of natural muscle fibers (i.e., high damping capacity and rapid mechanical recovery) but also high strength and toughness, higher even than that of many manmade and natural high-performance fibers,” they wrote.
“ … modeling results suggest that the excellent mechanical properties of the microbially produced UHMW titin fibers may originate from a unique inter-fibril pairing of folded Ig-like domains. Such inter-chain, non-covalent crosslinking through folded, stretchable domains has rarely been explored in either organic polymeric materials or other microbially produced fibers.”
Aside from its potential use in fancy clothes or protective armor, Sargent pointed out that the material could have biomedical applications as well. Because it’s nearly identical to the proteins found in muscle tissue, this synthetic material is presumably biocompatible and could therefore be a great material for sutures, tissue engineering, and so on. “The fiber’s highly desirable combination of mechanical properties, sustainable production process, and biodegradability make it an excellent candidate for environmentally friendly applications in a range of fields from biomedicine to commercial textiles (e.g., anti-ballistic materials, netting, sutures, and tissue engineering),” the team further stated.
“Its production can be cheap and scalable,” added Zhang. “It may enable many applications that people had previously thought about, but with natural muscle fibers.” Zhang’s research team doesn’t intend to stop with synthetic muscle fiber. The future will likely hold more unique materials enabled by their microbial synthesis strategy. Bowen, Cameron, and Zhang have filed a patent application based on the research.






