Make room, disulfide crosslinks. There’s a new kind of crosslink in proteins. Instead of an S–S bridge connecting two cysteine residues, it consists of an N–O–S bridge between a lysine and a cysteine. (The “N” comes from a lysine residue’s NH2 group, the “O” is an oxygen atom, and the “S” comes from a cysteine residue’s SH group.)
Like the S–S bridge, the N–O–S bridge can stabilize higher-order protein structures. Also, the formation of the N–O–S bridge appears to be chemically reversible. Accordingly, the N–O–S bridge suggests new regulatory possibilities for proteins.
The new crosslink was discovered by scientists based at Göttingen University, who anticipate that it may serve as a springboard for protein engineering as well as for drug design. Protein engineers may implement the lysine–cysteine motif into natural or designer proteins, and drug designers may find ways to target the motif with small-molecule inhibitors, antibodies, and nanobodies (or conjugates of these types of molecules).
The scientists discussed these possibilities in a paper that appeared May 5 in Nature, in an article titled, “A lysine–cysteine redox switch with an NOS bridge regulates enzyme function.” The article also describes how the scientists discovered the N–O–S bridge.
Led by Kai Tittmann, PhD, a professor of molecular enzymology at Göttingen, the scientists investigated a protein from Neisseria gonorrhoeae, a pathogen that causes gonorrhea. This disease is typically treated with antibiotics, but it threatens to become antibiotic resistant. To identify new treatments, the scientists studied the structure and mechanism of a protein that is a key player in carbon metabolism of the pathogen.
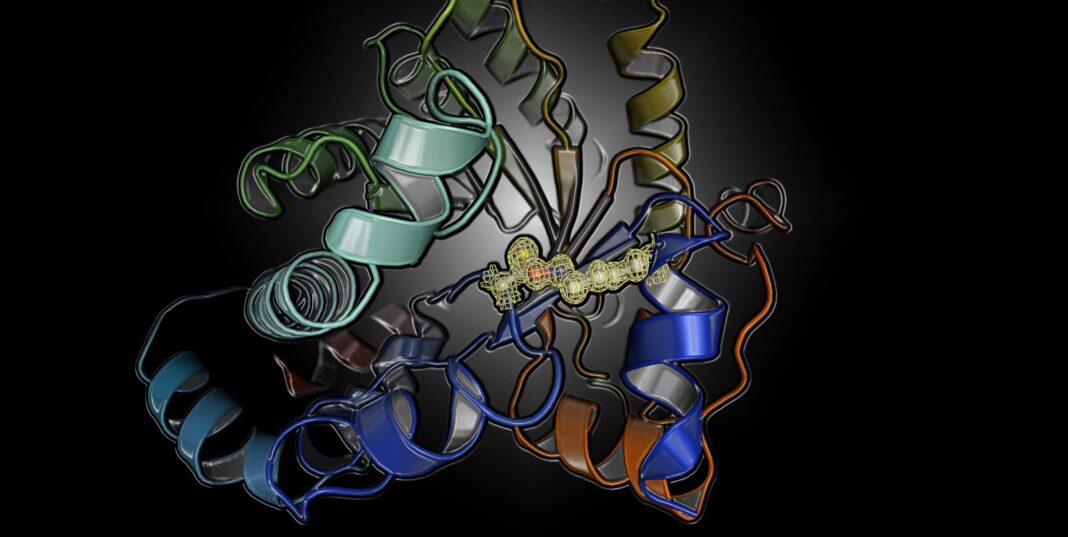
The scientists were surprised to learn that the protein can be switched on and off by oxidation and reduction (known as a redox switch). This finding led the scientists to suspect that the switching activity was due to the presence of disulfide crosslinks. When the scientists deciphered the X-ray structures of the protein in the “on” and “off” state at the DESY particle accelerator in Hamburg, Germany, they were hit by an even bigger surprise. The chemical nature of the switch was completely unknown: it is formed between a lysine and a cysteine amino acid with a bridging oxygen atom.
“X-ray structure analysis of the protein in the oxidized and reduced state reveals a loaded-spring mechanism that involves a structural relaxation upon redox activation, which is propagated from the allosteric redox switch at the protein surface to the active site in the protein interior,” the authors of the Nature article wrote. “This relaxation leads to a reconfiguration of key catalytic residues and elicits an increase in enzymatic activity of several orders of magnitude.”
Many repetitions of the experiments confirmed these results. Also, an analysis of the protein structure database further disclosed that there are many other proteins that very likely possess this switch, which apparently escaped earlier detection as the resolution of the protein structure analysis was insufficient to detect it for certain.
“I couldn’t believe my eyes,” said Tittmann of his first glimpse of the novel switch. “We thought initially that this must have formed artificially as a byproduct of the experimental process as this chemical entity was unknown.”
Tittmann and colleagues admitted that good fortune was on their side because the crystals they measured allowed the protein structure to be determined at extremely high resolution, meaning the novel switch couldn’t be missed. “The extensive screening for high-quality protein crystals has really paid off,” said Marie Wensien, a researcher in Tittmann’s laboratory and the first author of the paper. “I couldn’t be happier.”
The researchers believe the discovery of the novel protein switch will impact the life sciences in many ways. Besides enriching protein engineering and drug design, given that many proteins are redox-controlled, it could improve structure prediction.
“The spatial constraints for forming an NOS bridge are not as tight as those for a disulfide bond, as the lysine side chain is larger than that of a cysteine and thus allows for more flexibility in tethering protein chains intra- and intermolecularly,” the scientists added. “The involvement of lysine as part of the redox switch expands the regulatory tool set from that of disulfide–dithiol switches.”