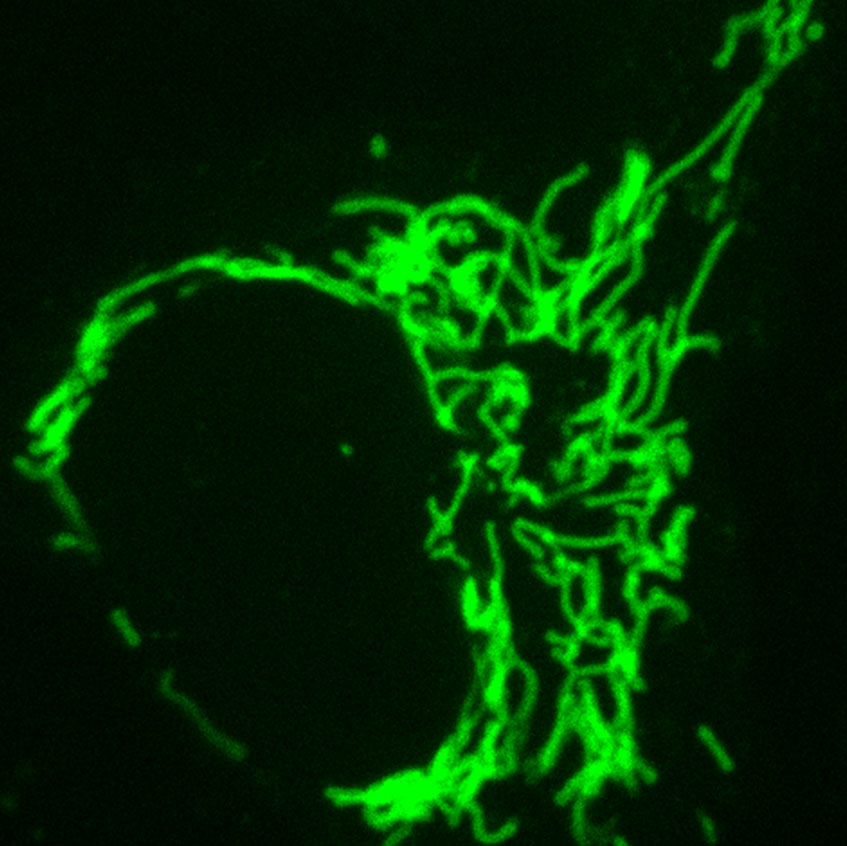
Stitching together complementarity-determining regions (CDRs) and single-chain variable fragment (scFv) scaffolds, scientists based at Colorado State University and Tokyo Institute of Technology have built what they call a frankenbody. This hybrid creation can bind a common epitope tag, the linear HA epitope tag. Most notably, it works in living systems, where it can add distinctive labels to diverse proteins. In a recent study, it enabled the multicolor visualization of HA-tagged nuclear, cytoplasmic, membrane, and mitochondrial proteins.
For many decades, scientists have cleverly exploited the selective tagging of natural antibodies to engineer antibody-based probes that can be used to purify and study different types of proteins within cells. One tried and true technique, epitope tagging, involves fusing an epitope to a protein of interest and using fluorescently labeled antibodies to make those proteins visible—but seldom in fixed, dead cells.
Now, a new approach has been developed by cross-disciplinary team of researchers. Some of the researchers come from a lab led by Colorado State University’s Tim Stasevich; others, from a lab led by Tokyo Tech’s Hiroshi Kimura. Together, they have developed a genetically encoded probe expressly designed to work in living cells.
Details appeared July 3 in the journal Nature Communications, in an article titled, “A genetically encoded probe for imaging nascent and mature HA-tagged proteins in vivo.”
“[We used the HA frankenbody [to track] single HA-tagged histones in U2OS cells and single mRNA translation dynamics in both U2OS cells and neurons,” the article’s authors wrote. “Together with the SunTag, we also track two mRNA species simultaneously to demonstrate comparative single-molecule studies of translation can now be done with genetically encoded tools alone. Finally, we use the HA frankenbody to precisely quantify the expression of HA-tagged proteins in developing zebrafish embryos.”
Like stitching new limbs on a body, the scientists have taken the binding regions of a normal antibody, the “sticky parts,” and grafted them to a different scaffold that remains stable in live cells but retains the specificity of the antibody.
“We’re interested in intracellular antibodies because you can use them as imaging reagents in a live cell,” said Stasevich. “You don’t need a tag, like a green fluorescent protein, because instead you have this fluorescent antibody that will bind to your protein that you want to visualize.”
The new probe would be a useful complement to the green fluorescent protein, a widespread biochemistry tool and subject of a Nobel Prize that involves genetically fusing a light-up green tag to a protein of interest. However, the GFP is limited by its relatively large size and the time it takes to fluoresce; with the CSU researchers’ new probe, the tag is smaller and becomes fluorescent faster, so the “birth” of a protein of interest can be captured in real time.
With the goal of making their tool immediately useful, the scientists designed their probe to work with the classic HA tag. HA is a widely used small linear epitope tag that’s derived from a portion of the human influenza virus protein hemagglutinin.
“For the longest time, people have been looking at HA-tagged proteins in fixed, dead cells,” Stasevich said. “Now we can image the dynamics of those proteins in live cells.”
The scientists in Stasevich’s lab are particularly interested in studying RNA translation, and they plan to use their new system to more easily design new RNA imaging experiments.
According to Ning Zhao, a postdoctoral research in Stasevich’s lab and the current study’s first author, the HA tag is tiny—a chain of just nine amino acids—and the probe is genetically encoded on a plasmid that can be easily transferred into a cell. This is in contrast to traditional antibodies, which can cost a lab several hundreds of dollars per order, suffer from lot-to-lot variability, and are difficult to get into cells. The new probe from Stasevich’s team, therefore, provides a low-cost solution for protein and RNA translation imaging.
“We have several new imaging reagents in the works that build off of this success,” Stasevich pointed out. “So, I see great things ahead.”