Therapeutic paradigms in the SARS-CoV-2 pandemic have primarily focused on the virus. A new study by Italian scientists shifts this focus on to the human target cell instead. The researchers use a couple of single-strand DNA molecules to mask the key site on the human ACE2 receptor that binds to the SARS-CoV-2 spike protein, effectively barricading the door that the coronavirus uses to enter human cells.
Published in the journal Pharmacological Research, the article titled “DNA aptamers masking angiotensin converting enzyme 2 as an innovative way to treat SARS- CoV-2 pandemic,” describes this new precision strategy to block the coronavirus from infecting human cells. The findings are the basis of a new drug for which a patent has been filed recently.
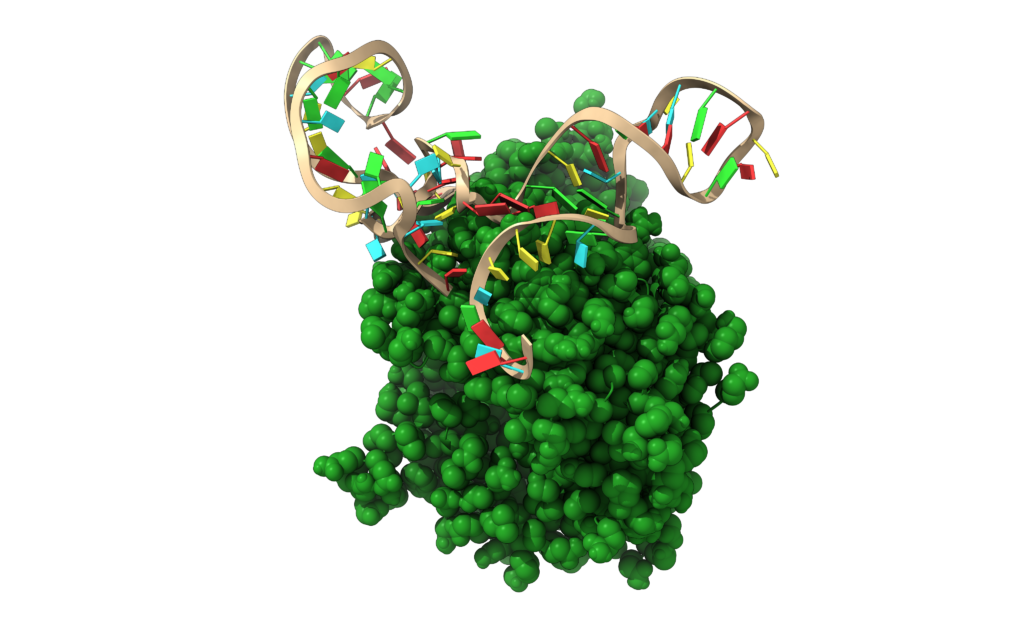
“Our approach brings a significant innovation to the therapeutic paradigm of the SARS-CoV-2 pandemic by protecting the target cell instead of focusing on the virus; this is particularly attractive in light of the increasing number of viral mutants that may potentially escape the currently developed immune-mediated neutralization strategies,” the authors note.
The study led by Paolo Ciana, PhD, professor of pharmacology at the University of Milan, Vincenzo Lionetti, PhD, professor of anesthesiology at the Scuola Superiore Sant’Anna, and Angelo Reggiani, PhD, senior researcher and principal investigator in Pharmacology at the Italian Institute of Technology, explores the possibility of preventing infection by any SARS-CoV-2 variant by blocking the docking site of the virus on the human ACE2 receptor.
“Thanks to this study, it will now be possible to develop a new precision therapeutic approach to prevent COVID-19 infection in a severe form, without stimulating the immune system or having important side effects related to the most popular drugs consisting of monoclonal antibodies or other therapeutic proteins. In this sense, in fact, the potential toxicities of nucleic acids as drugs are far less than other innovative drugs such as monoclonal antibodies or other therapeutic proteins”, they commented.
To accomplish this, the researchers applied a new procedure called SELEX (systematic evolution of ligands by exponential enrichment) to identify two single-strand DNA molecules (aptamers) that interact specifically with the region surrounding the key residue in the human ACE2 protein (lysine353) that interacts with the SARS-CoV-2 spike protein residue (asparagine501), making the human docking site inaccessible to the spike protein of any coronavirus.
SELEX is an in vitro selection procedure based on repeated target-driven PCR (Polymerase chain reaction) amplification. The method starts with a library of random single strand DNA or RNA oligonucleotides and enriches the amplification products with a pool of oligonucleotides with high affinity for the molecular target used for the selection.
“Applying in vitro and in silico approaches, we demonstrated that these aptamers could generate a steric hindrance on ACE2, thus preventing the binding of the cleaved S1 subunit SARS-CoV-2 S to the cellular receptor regardless of the viral variant and inhibiting the infection of pseudoviral particles carrying the S protein from SARS-CoV-2,” the authors note.