Through research that could impact on the future study of diseases ranging from stroke to dementia, scientists at the University of California, San Francisco (UCSF), and colleagues have catalogued all the cells that form the blood vessels of the human brain, along with their locations and the genes transcribed in each. The atlas characterizes more than 40 previously unknown cell types, including a population of immune cells whose communication with the brain’s vascular cells contributes to the bleeding of a hemorrhagic stroke. This devastating form of stroke—which is fatal in about half of cases—accounts for 10-15% of all strokes in the U.S., mostly among younger people.
The team suggests that their reported findings will serve as a foundation for new research on brain vasculature globally. “This research gives us the map and the list of targets to start developing new therapies that could change the way we treat a lot of cerebrovascular diseases,” said Ethan Winkler, MD, PhD, a neurosurgeon and research associate at the UCSF Weill Institute for Neurosciences and one of the lead authors of the study, which is published in Science, and titled “A single-cell atlas of the normal and malformed human brain vasculature,” in which they concluded, “This atlas has important implications for neuroscience and clinical medicine.”
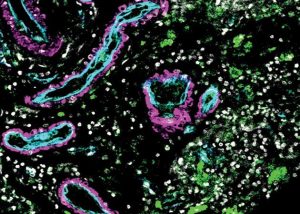
The cerebrovasculature is tasked with ensuring the delivery of oxygen and nutrients to the brain, the removal of by-products of brain metabolism, and preventing the entry of circulating toxins. And as the the authors pointed out, “Interruptions in cerebral blood flow or structural compromise and hemorrhage lead to stroke, which is a leading cause of death and disability worldwide.”
For their reported study, the researchers analyzed cells in arteriovenous malformations, or AVMs. These tangles of poorly formed arteries in the brain are often the source of a hemorrhagic stroke. They compared the AVMs with samples of normal brain vasculature from five volunteers who were already undergoing brain surgery for epilepsy. The study was headed by Adib Abla, MD, associate professor of neurological surgery and Daniel Lim, MD, PhD, professor of neurological surgery, who are both members of the UCSF Weill Institute for Neuroscience, along with Tomasz Nowakowski, PhD.
Some of the 44 samples of AVM tissue, acquired during surgeries performed by Abla, who is Chief of Neurological Surgery, had been removed from the patient’s brain while still intact, and other samples were only removed after they had started to bleed. Analyzing three varieties of tissue—normal, intact AVMs, and AVMs that had bled—allowed the researchers to get a fuller picture of differences between how the cells function normally and in different states of disease.
In collaboration with the Cerebrovascular Research Center, the team used single-cell mRNA sequencing on more than 180,000 cells to determine which genes were being expressed in the differing samples, and matched gene expression with a cell’s location.“We profiled transcriptomes of 181,388 cells to define a cell atlas of the adult human cerebrovasculature, including endothelial cell molecular signatures with arteriovenous segmentation and expanded perivascular cell diversity,” the authors explained. Chang Kim, a graduate student in bioinformatics at UCSF and co-lead author of the study, then developed computer analyses that compared gene expression in the normal and diseased cells.
The results revealed not only a variety of new cell types, but a population of immune cells that appear to communicate with smooth muscle cells in the diseased arteries and weaken them, resulting in a stroke. Scientists have suspected that the immune system is activated by malformations like AVMs. However, Nowakowski said, “without this study, we wouldn’t be able to pinpoint this very specific population of cells in the blood that might be the key drivers of disease progression.” As the authors further noted, “We identified conservation of endothelial molecular zonations essential to arteriovenous phenotypic change and expanded cellular diversity of brain perivascular cells, including fibromyocytes not previously identified in the cerebrovasculature.” Identifying these specific immune cells completely changes how researchers can think about treating this sort of vascular disease, he added. If the cells circulate in the blood, it may be possible to reduce stroke risk by modulating the immune system. “This opens up huge therapeutic potential,” said Nowakowski.
That potential extends beyond stroke. The map may help researchers investigate any neurovascular disease, including dementia. “Many forms of dementia, including Alzheimer’s, appear to have a vascular underpinning,” said Lim. “We need an atlas like this to better understand how changes in the vasculature can contribute to the loss of cognition and memory.”
“This work was a really a beautiful collaboration between surgeon-scientists and molecular biologists, occurring in a place with incredible access to clinical specimens,” continued Lim, who further noted that while many institutions don’t have access to all of these critical resources, they will have access to the dataset from this study. Nowakowski believes that this information will allow researchers across the world to perform much less expensive analyses on large numbers of patients, which is the only way to get a fuller picture of how vascular diseases operate.“Understanding cerebrovascular disease at the cellular and molecular level will take the work of many researchers into new directions,” Lim further noted.
The team’s study contributes to the Human Cell Atlas, an international effort to create cell reference maps for the entire body. Nowakowski calls these atlases a “periodic table of cell types.” Just as the chemical periodic table organizes elements into a structure that allows chemists to draw relationships between them based on where they appear in the table, human cell atlases reveal the locations of cells in the body and the resulting interactions between them.
While there is a lot of work taking place around the world to generate these atlases for different organs and tissues, many of them only map the geographic locations of cells. The comparison of normal and abnormal cells in this research takes it to a higher level, providing extremely refined guidance for drug development.
“Our study really demonstrates how a cell atlas can be utilized,” Nowakowski said. “With our ‘periodic table’ as a reference, we can start asking which cells might go wrong in disease and very precisely target those cells for therapy.”
Noting potential limitations of their study, the authors concluded, “We recognize that this atlas represents only a first step toward a comprehensive census of the human cerebrovasculature … Additional work will also be needed to ascertain distinctions between cell types and cell states, such as transient or metabolic variations. Nonetheless, our results should inform future studies in other brain regions or cerebrovascular diseases to accelerate mechanistic understanding and therapeutic targeting of the human cerebrovasculature.”