Some things that come in threes are seen in a positive light: the Triple Crown of horse racing, the Three Musketeers, and hockey’s “hat trick.” But threes also have their dark side, as evidenced by the so-called tripledemic—the unprecedented concurrence this winter of three major respiratory viruses: respiratory syncytial virus (RSV), seasonal influenza, and COVID-19. At times, the tripledemic has threatened to overwhelm the healthcare system.
By early winter, RSV, flu, and COVID-19 were surging more or less simultaneously. Might such multipronged attacks become the norm? “Most certainly COVID-19 is not going away,” says Steven J. Lawrence, MD, professor of medicine, Division of Infectious Diseases, Washington University School of Medicine. “However, many believe that once the variants settle down, there will be a more seasonal pattern. The problem is that when a new variant appears, it begins to infect a lot of people no matter what time of year it is. However, we do see some seasonality with COVID-19.”
Lawrence also suggests that the tripledemic pattern may be here to stay: “It is likely that this is going to be the new normal with each year seeing the co-circulation and seasonality of RSV, flu, and COVID-19.”
Surveillance and testing
Identifying which virus is causing an infection is not only critical to appropriate medical management, but also necessary for properly tracking outbreaks. A global network has already been established for tracking influenza. “There are reference sites that have been around a long time, such as doctors’ offices and clinics,” Lawrence notes. “Each year, members send their flu isolates into a central repository for analysis. Thus, we are tracking not only flu numbers but also strains and the mutations that are occurring.” An updated weekly U.S. influenza surveillance report (FluView) is available from the Centers for Disease Control and Prevention (CDC).
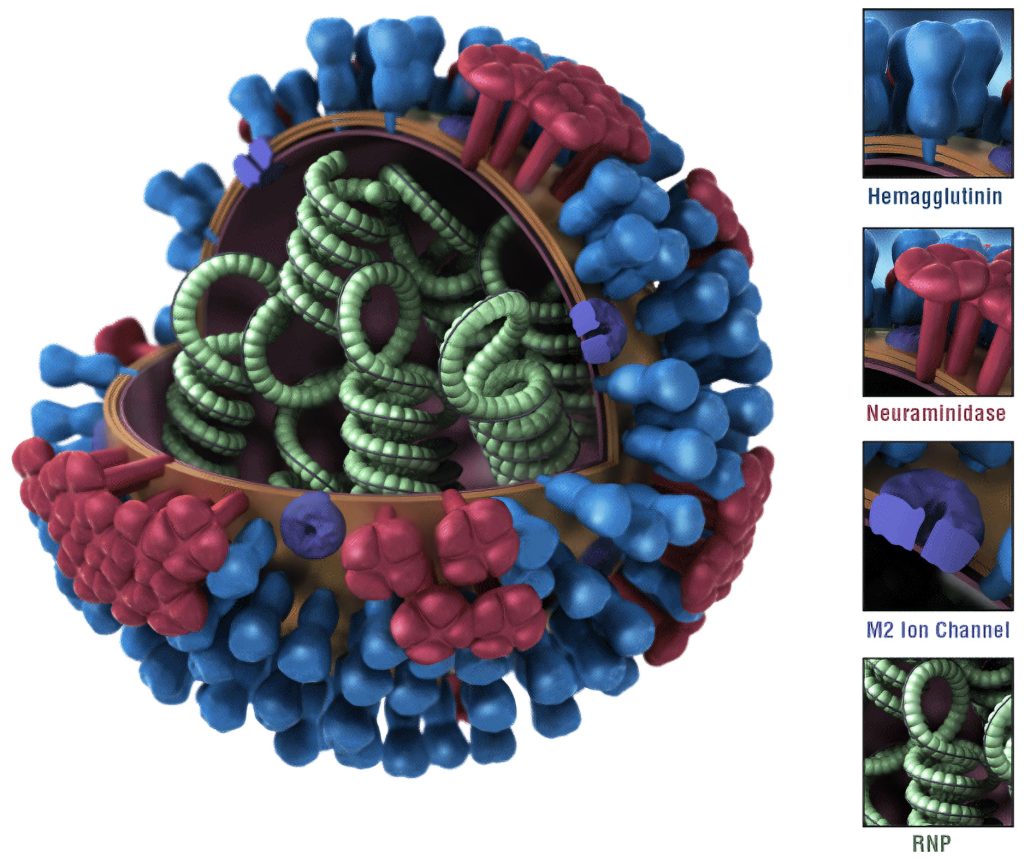
Lawrence indicates that COVID-19 surveillance lags behind but is becoming more robust in tracking numbers of cases and in using sophisticated genomic analyses of SARS-CoV-2 to identify which variants are circulating. He points out, “However, RSV hasn’t caught up in surveillance with the others. There are pockets around the country performing RSV surveillance. They don’t have quite the robustness that has been established for flu and COVID-19, but they still provide insights.”
As for testing to diagnose viruses, PCR testing remains the gold standard for identifying and differentiating each virus. “During flu season, multiplex tests performed by healthcare providers are valuable to distinguish each virus,” Lawrence elaborates. “While home COVID-19 tests are widely available, they are not as available for other viruses. But since there is overlap among symptoms, and since there are specific treatments for COVID-19 and flu, it is important to determine which virus is causing infections.”
According to Lawrence, there are two major forms of therapy for COVID-19. The first form involves the administration of drugs that stop viral replication. These drugs include Paxlovid (nirmatrelvir/ritonavir), Veklury (remdesivir), and Lagevrio (molnupiravir). The second form involves monoclonal antibodies such as Evusheld (tixagevimab/cilgavimab).
For pre-exposure prophylaxis, Evusheld is becoming less effective against the newer COVID-19 variants. Bebtelovimab, another monoclonal antibody, no longer works against the current dominant strain (Omicron variant BQ1.1). Accordingly, the Food and Drug Administration has pulled bebtelovimab’s emergency use authorization.
Lawrence stresses that it is important for patients to seek flu treatment, especially if they are high-risk individuals. The CDC reports that all viruses evaluated were susceptible to the influenza antivirals Tamiflu (oseltamivir), Rapivab (peramivir), Relenza (zanamivir), and Xofluza (baloxavir).
Faster and more potent flu vaccines
Keeping up with fast-mutating viruses is no easy feat. For example, the rapid emergence of a veritable smorgasbord of SARS-CoV-2 variants has challenged vaccine developers. Fortunately, these developers have been able to take advantage of mRNA technology, which continues to improve and expedite the appearance of COVID-19 vaccines. And now mRNA technology is being used to engineer improved flu vaccines.
Scientists at the Washington School of Medicine are conducting a Phase II study to assess a new mRNA flu vaccine developed by Moderna (mRNA-1010). “Our study is designed to examine in detail the immunologic response of this mRNA vaccine as compared to the standard flu vaccine,” reports Rachel Presti, MD, PhD, associate professor of medicine and medical director of the institution’s Infectious Diseases Clinical Research Unit.
According to Presti, one major challenge of the current flu vaccines is that they sometimes target the wrong strains. “Every year, the CDC has data for how well the flu vaccine strains chosen matched the strains that actually circulated,” she notes. “Because traditional flu vaccines take longer to prepare, we have to choose strains very early in the year. Even when the best available data is used, incorrect strains are sometimes chosen to be in the vaccine.
“What is exciting about mRNA vaccines is that the speed of preparing them allows us to wait, for example, until late summer to see what is circulating in the world. Those few extra months provide better current data to select more accurately the best strains to use.”
Although the technology to produce mRNA vaccines is still new, Presti feels that it could be the wave of the future, especially against viruses. “For viruses where we know what antigens we need to target, it may be of more use,” she points out. “For bacterial pathogens that may require targeting of sugar structures, for example, mRNA vaccines may not be the solution. But I do think more and more mRNA vaccines will be developed—especially against epidemic-like viruses, where we need to respond rapidly.”
RSV immunity gap?
One reason RSV has surged this year may relate to an “immunity gap,” according to Dan Rocca, PhD, a research leader at Charles River Laboratories. “During the pandemic, the safety measures such as lockdowns, mask wearing, and social distancing which kept us protected from catching COVID-19 also protected us from the large variety of other respiratory viruses such as influenza and RSV,” he says. “It is likely that many of these preventative COVID-19 measures led to a lack of exposure to the virus and an ‘immunity gap’ where the usual immune response to existing RSV strains that populations build over time was subsequently greatly diminished.”
Several therapies are being used to help the most susceptible or most at-risk patients avoid hospitalization. “Palivizumab [sold under the brand name Synagis], for example, is a monoclonal antibody therapy directed specifically at RSV,” details Sandy Kimber, PhD, group leader of the Portishead UK Infection Team at Charles River. “Ribavirin [sold under brand names such as Moderiba, Virazole, and Ribasphere] is another antiviral inhibitor therapy that is effective against RSV, but it isn’t used in children due to long-term safety concerns.
“More RSV antiviral inhibitors that prevent viral entry and replication are in development, as well as therapeutic antibodies to limit infection. One such example is the recently approved nersivemab from AstraZeneca/Sanofi [sold under the brand name Beyfortus]—a long-lived monoclonal antibody of demonstrated superiority that has cost benefits over current acute therapies.”
RSV maternal vaccine
Although the development of vaccines against RSV has lagged behind the development of vaccines against flu and COVID-19, a new maternal vaccine candidate against RSV is undergoing clinical trials. One of the scientists working on these trials is Carol Kao, MD, assistant professor of pediatrics, Washington University School of Medicine.
“RSV is the number one cause of infant hospitalizations in the United States,” she says. “Worldwide, it causes over 30 million infections in children less than five years of age and is a leading cause of death in infants. RSV prevention in infants is a huge unmet need; there are no widely available preventative measures or treatments for RSV illness. All we can really do is support babies with intravenous fluids or supplemental oxygen until they get better.”
She adds, however, that the new RSV vaccine candidate looks promising. “This would be the first RSV vaccine approved,” she remarks. “So, this is a very exciting time.”
The new vaccine candidate is called RSVpreF, and it is being developed by Pfizer. The company says that RSVpreF is a bivalent vaccine candidate that is composed of equal amounts of recombinant RSV prefusion F from subgroups A and B.
“Given that young infants are the most severely affected by RSV, a maternal vaccine given during pregnancy would allow for infants to be protected right off the bat, during their most vulnerable early months of life,” Kao explains. “This is similar to why we give influenza, COVID-19, and Tdap (pertussis) vaccines to women during pregnancy.”

Pfizer recently announced positive top-line data from MATISSE (MATernal Immunization Study for Safety and Efficacy), a Phase III, randomized, double-blinded, placebo-controlled trial that started in June 2020 and that has spanned multiple RSV seasons in both the northern and southern hemispheres. Pregnant women were vaccinated in their late second to third trimester of pregnancy, followed for six months after delivery, and their infants were followed for at least one to two years. Kao reports, “Preliminary study results, which included 7,400 pregnant people and infants, showed that the vaccine was well tolerated and 82% effective at preventing severe lower respiratory tract infections in babies for the first three months of life and 70% effective for the first six months of life.”
Advancing analytics and surveillance
Even amid the tripledemic, scientists continue to improve the analysis of vaccines as well as the surveillance of viral variants. For example, scientists at Sciex and Precision NanoSystems are collaborating to develop highly analytical tests to support the development of mRNA vaccines and other products.

Precision NanoSystems
“The established and ideal carriers for mRNA-based medicines, including certain COVID-19 vaccines and mRNA therapeutics, are lipid nanoparticles (LNPs),” asserts Kerstin Pohl, senior manager of cell and gene therapy and nucleic acids, SCIEX. Recent studies, however, have identified an impurity that originates from ionizable lipids, a key component of LNPs. “Ionizable lipids are susceptible to chemical alterations—predominantly oxidation,” Pohl notes. “Site-specific oxidation can lead to the formation of reactive lipid species that bind to mRNA to form mRNA-lipid adducts. These adducts, even in tiny amounts, can prevent mRNA-triggered protein expression, reducing drug efficacy.”

Sciex has developed a new electron-activated dissociation (EAD) mass spectrometry approach that can analyze LNPs and lipid raw materials at very low abundance, revealing exact oxidation sites. According to Adam Crowe, PhD, manager of analytical development, Precision NanoSystems, advanced analytics can help biopharmaceutical companies manage the challenges of mRNA lipidation. He adds that these companies are increasingly requiring support from contract development and manufacturing organizations.
“Direct monitoring of adduct formation on mRNA via ion-pair reversed-phase high-performance liquid chromatography with ultraviolet spectroscopy detection and mass spectrometry has proven effective,” Crowe states. “However, significant expertise is required to optimize methods, generate controls, and ensure assay robustness.”
Improvements in next-generation sequencing are also tightening the surveillance of variants. Ramin Khaksar, PhD, COO and CSO at Clear Labs, says that such improvements are evident in his company’s surveillance solution: “It is a uniquely complete end-to-end, near-real-time solution that integrates software, hardware, bioinformatics, analytics, and reporting with a single touch point. This integrated solution enables NGS applications to be more adaptable to a variety of laboratories without barriers from lab and computer infrastructure or access to skilled scientists and bioinformaticians.”
The tally
At the end of 2022, the CDC released estimates that it had gathered for the flu season up to that point: 20 million illnesses, 210,000 hospitalizations, and 13,000 deaths. The good news was that the CDC also indicated that the majority of flu viruses tested were antigenically similar to those included in the current year’s vaccine. At the time, 44% of U.S. counties were experiencing medium to high COVID-19 levels in each community. (To see current results, visit the CDC’s COVID Data Tracker, an online resource.)
Although experts warn that the tripledemic trend will likely continue in subsequent seasons, scientists continue fighting back by developing improved antiviral vaccines and treatments as well as more sophisticated and accurate analytical and surveillance technologies.