Source: Cell
It delivers a one-two punch. “It” is a small-molecule drug that incorporates an antibiotic and an antigenic epitope. The antibiotic directly kills gram-negative bacteria, and the antigenic epitope induces a targeted immune response. Linked together, these components constitute a deadly duo, one that may defeat antibiotic-resistant strains of Pseudomonas aeruginosa, which causes pneumonia and sepsis, and Escherichia coli, which causes food-borne illnesses, urinary tract infections, and many other illnesses.
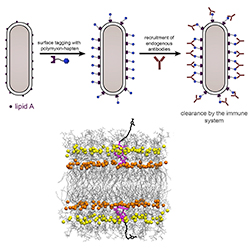
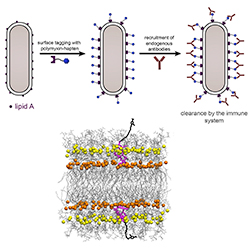
Several promising antibiotic-antigen conjugate leads have been developed by scientists based at Lehigh University. These scientists started by demonstrating a method of labeling the surface of gram-positive bacteria with antigenic epitopes, the part of a foreign substance that is recognized by the immune system. The scientists then followed up on these efforts by assembling antibiotic-antigen conjugates against gram-negative bacteria.
Each of these conjugates incorporates polymyxin B (PMB), an antibiotic that inherently attaches to the surface of gram-negative pathogens. The conjugates also carry antigenic epitopes that recruit antibodies and, potentially, reengage components of the immune system in the fight against bacterial infection.
Details appeared July 5 in the journal Cell Chemical Biology, in an article entitled, “Synthetic Immunotherapeutics against Gram-Negative Pathogens.” As the title emphasizes, the idea is to expand the utility of immunotherapy, which to date has been used to destroy cancer cells. Essentially, the article describes how immunotherapy may also target bacteria. This kind of immunotherapy could be called bacterial immunotherapy or immunobiotics.
“We demonstrated the specific killing of bacteria by our agent in the presence of human serum,” the article’s authors write. “By enlisting the immune system, these agents have the potential to pave the way for a potent antimicrobial modality.”
The PMB-antigen conjugates successfully recruited the antibodies found in human serum, giving an indication that they may be capable of harnessing the immune system to eliminate dangerous, disease-causing bacteria.
“To target these bacteria, we turned to an old class of antibiotics known as colistin,” explains Marcos Pires, an associate professor of chemistry at Lehigh and the current study’s senior author. “Colistin is a last-resort antibiotic. It just so happens that it destroys bacteria by landing on its surface. We modified colistin with an agent that attracts antibodies onto the surface of the bacteria and built a compound that both directly kills bacteria and at the same time induces an immune response.”
Pires and his team used computational biophysics to learn how antibiotics permeate bacterial membranes and target bacteria for destruction. “We established that the spacer length played a significant role in hapten display within the bacterial cell surface,” the article’s authors detailed, “a result that was confirmed both experimentally and via molecular dynamics simulations.”
“During the optimization stage,” notes Pires, “we modeled how the surface composition of the bacteria may hinder or assist in the immune system activation.”
“In the future, we plan to expand our in vivo studies to complex animals to establish the suitability of this class of molecules to fight infections,” the article concludes. “Moreover, we will explore how our strategy can be used to induce the grafting of exogenous haptens onto bacterial cell surfaces with the goal of providing finer control on antibody levels.”