Sponsored content brought to you by

Despite the significant technological advances in life sciences to decipher cellular pathways and manipulate cells with unprecedented precision, scientists often settle for the circa 1960s Ficoll® density gradient centrifugation–based method for cell separation and debulking due to a lack of viable alternatives. This decades-old non-scalable technology is labor-intensive and operator variable.
MicroMedicine’s Sorterra™ is a new, automated, label-free approach to cell isolation and concentration. By leveraging patented microfluidic technology, no centrifugation, beads, antibodies, or density gradient reagents are required for the separation. Sorterra provides a reliable sample preparation for downstream functional assays in clinical research laboratories.
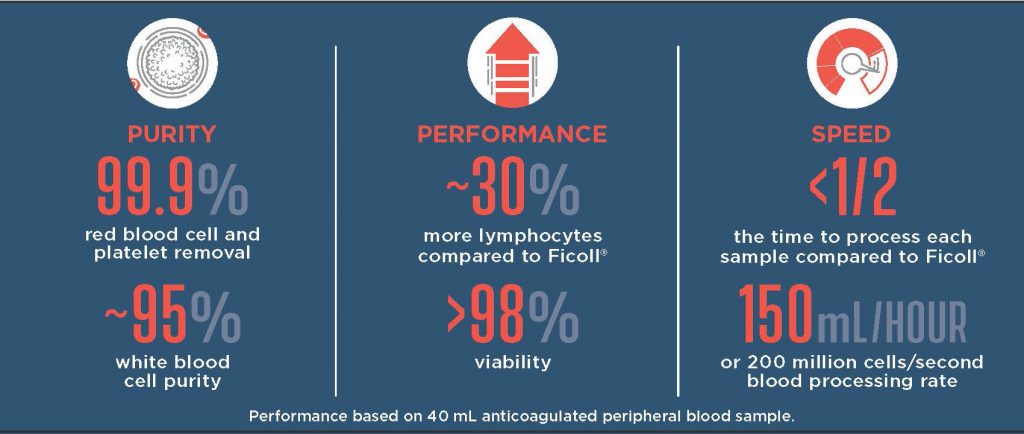
When processing whole blood samples, success is maximizing the number and quality of target cells and minimizing contaminating cells, with a reliable and consistent process. While Ficoll relies on the skill and dexterity of the operator, the Sorterra automated system significantly reduces operator-to-operator variability and recovers more white blood cells compared to Ficoll.
“The system recovers twice as many nucleated cells in half the time, leading to less input blood required to conduct the downstream assays, and significant time savings,” said John Powderly, MD, founder of Carolina BioOncology and an early adopter of the Sorterra system.
 The intuitive user interface provides step-by-step instructions on how to load the microfluidic set, add the sample of 3–75 mL anticoagulated blood, and dilute with saline, all requiring <5 minutes of hands-on time. The automated system then processes the sample through the microfluidic chip requiring less than half the processing time as Ficoll.
The intuitive user interface provides step-by-step instructions on how to load the microfluidic set, add the sample of 3–75 mL anticoagulated blood, and dilute with saline, all requiring <5 minutes of hands-on time. The automated system then processes the sample through the microfluidic chip requiring less than half the processing time as Ficoll.
In recent comparative studies between the automated microfluidic cell separation system and conventional density gradient cell separation with Ficoll, anticoagulated blood samples were obtained from healthy donors and processed within 8 hours. Samples were split, and 40 mL was processed through each method. Sorterra repeatedly and consistently yielded 25% more PBMCs and a 10x purer isolate compared to Ficoll. Furthermore, Sorterra yielded 93% of the granulocytes, providing a leukocyte isolate that was consistent with the leukocyte composition in blood, while Ficoll typically only retains peripheral blood mononuclear cells (PBMCs). In a similar study, lymphocyte functionality was assessed and the microfluidic approach was found to have equivalent secretory functionality of IFNγ, IgG, and IgM, as measured by ELISpot.
Another significant finding when comparing the two technologies was that approximately 93% of Ficoll isolated monocytes were positive for platelet marker CD41/61. Of these, 88% were positive for platelet activation marker CD62p. Contrastingly, only 1% of Sorterra isolated monocytes were positive for platelet marker CD41/61. Of these, an undetectable amount were positive for platelet activation marker CD62p.
 MicroMedicine’s FDA-listed Class I medical device offers reliable sample preparation for downstream functional assays and has the potential to transform academic and clinical research by automating a variable and labor-intensive process that adds to workflow complications and cost. For clinical research in clinical trials, this system could be integrated at the draw site, where existing phlebotomists could easily be trained to perform the separations. When scaled across multiple sites, workflow efficiency and consistency improvements would multiply.
MicroMedicine’s FDA-listed Class I medical device offers reliable sample preparation for downstream functional assays and has the potential to transform academic and clinical research by automating a variable and labor-intensive process that adds to workflow complications and cost. For clinical research in clinical trials, this system could be integrated at the draw site, where existing phlebotomists could easily be trained to perform the separations. When scaled across multiple sites, workflow efficiency and consistency improvements would multiply.
“MicroMedicine’s mission is to use the precise fluid and cell handling enabled by microfluidics to create better tools for cell sorting. We are very excited to offer this new and improved solution,” stated Ravi Kapur, PhD, co-founder and CEO of MicroMedicine.
Learn more: www.micromedicine.com.


