A few days before starting SynBioBeta 2020, the event’s organizers distributed a mass email that carried an arrestingly blunt subject line: “digital events suck.” This was a bold assertion, given that the event was to be held virtually, as are many events these days. But the email’s author, John Cumbers, PhD, founder and CEO of SynBioBeta, explained that the organization intended to change up the virtual conference format. SynBioBeta, he insisted, had done everything possible to ensure that its virtual event, the “Global Synthetic Biology Summit,” would amount to more than a collection of Zoom calls. It would, instead, instill a sense of community among people who “share a passion for using biology to build a better, more sustainable universe.”
Cumbers’ community-minded message was fitting, given that SynBioBeta calls itself a synthetic biology (synbio) “innovation network.” Even better, the message was borne out by the event, which justified the innovation theme throughout, no matter the topic under discussion—food, human health, COVID-19, or space travel. The innovation theme was evident even in details of staging. For example, when Cumbers delivered his introductory address, virtual attendees might not have guessed that he was speaking from his basement. He appeared before a synbio-themed backdrop of MycoComposite panels. (The panels, provided by Ecovative Design, were fabricated from mushroom-based materials that can substitute for plastics.)

Marching to a different drum
Although 2020 has walloped many industries, Cumbers noted that synbio had been able to buck the trend: 56 startups had raised more than $3 billion over the past six months, compared to $1.9 billion in all of 2019. Besides addressing investment and bioeconomy issues, speakers at the three-day event discussed sustainable technologies and biopharma applications. A central message at the event was that synbio could prevent or even reverse the ecological damage and resource depletion associated with conventional technologies.
This year’s “best new product” was awarded to Conagen and its CEO, Oliver Yu, PhD. Conagen—famous for making nature-inspired molecules such as noncaloric food sweeteners—moved into improving infant health by commercializing non-GMO human milk oligosaccharide and human lactoferrin.
The week before receiving an award so momentous as to need no discussion here, Jennifer Doudna, PhD, of the University of California, Berkeley, and the Howard Hughes Medical Institute was named this year’s Pioneer in Synthetic Biology at SynBioBeta. When Doudna accepted the award, she mentioned that the Innovative Genomics Institute (IGI), a nonprofit research organization that she founded in 2014, has established a COVID-19 diagnostic testing laboratory on the UC Berkeley campus.
The laboratory is using an established RT-PCR-based test that can detect SARS-CoV-2 RNA. At the same time, IGI scientists are working toward using CRISPR proteins to make inexpensive diagnostics that could be used at home.
Doudna discussed the importance of prioritizing the affordability and accessibility of CRISPR-based health applications in the future. Doudna emphasized that science is highly interconnected and that scientists have to think about “how it affects our world and fellow human beings.” Science and fundamental discovery work, she added, go “hand in hand” with the development of “practical solutions to real-world problems.”
Lighting into COVID-19
In early February, COVID-19 researchers in China were working to immunize mice. Eight mice that became seropositive received a police escort that took them 1,200 miles across China to a laboratory in Nanjing belonging to the biotechnology company GenScript. Why the long trek? The laboratory was equipped with an optofluidic technology called Beacon. Developed by Berkeley Lights, Beacon is a means of quickly analyzing and isolating selected cells.
At the GenScript laboratory, Beacon screened immune cells from the mice and identified antibodies capable of interfering with SARS-CoV-2 binding. From start to finish, the analysis took just 24 hours, not the three months it would have taken with traditional technology.
Just a month later, Beacon was advancing COVID-19 research yet again, this time at the Vanderbilt Vaccine Center, where director James E. Crowe, MD, needed to analyze blood samples from recovered COVID-19 patients. The samples, which had been sent by the Defense Advanced Research Projects Agency (DARPA), were run on the Beacon platform to develop an antibody-based therapeutic treatment. Over 500 antibodies were identified in a single day, one of which is present in an AstraZeneca antibody cocktail that is currently entering a Phase III trial.
A technology that moves single cells with light is responsible for these projects moving so quickly. Developed by Ming Wu, PhD, professor of electrical engineering and computer sciences at University of California, Berkeley, the technology formed the basis of Berkeley Lights, which was founded by Wu in 2011.
When Berkeley Lights went public in July, Cumbers wrote in Forbes that the company had a “wildly successful IPO that raised $205 million,” more than double the company’s $100-million filing. The company is currently valued at around $4 billion.
The technology’s applications stretch far beyond COVID-19. Right now, the company works in antibody therapeutics, synthetic biology, and cell therapies. A commonality between Berkeley Lights’ customers is the need to find the cell that will lead to the best cell-based product. To do that, the customers use Beacon to isolate individual cells and subject them to analysis. After delivering functional results that identify the best cells, Beacon can direct them to additional processing steps.

The platform uses its opto-electropositioning (OEP) technology, a combination of optics and nanofluidics that uses light to move cells onto OptoSelect chips. Light and semiconductor technology combine to move single cells into isolated chambers called NanoPens. The entryway into the NanoPen is about the width of a human hair, the volume a nanoliter. Each cell is isolated and cultured as a single cell in NanoPens, establishing a clonal cell culture that can then be selected and exported.
The platform is not cheap. Eric Hobbs, PhD, CEO of Berkeley Lights, tells GEN that the Beacon platform costs “millions of dollars.” Eight of the top 10 pharma companies in the world have the platform, and some startup companies are becoming customers as well.

What does this have to do with synbio? The Beacon platform could be used to accelerate the development and commercialization of cell-based products. According to Hobbs, such products could be manufactured sustainably and at price points low enough to ensure that everyone can benefit. The big cell-based market areas where the company’s technology could be applicable are biotherapeutics, new and sustainable foods, and industrial materials (such as the wall of mushroom bricks that Cumbers had behind him when he opened the conference).
Last year, Berkeley Lights signed a $150-million deal with Ginkgo Bioworks, a leading synbio company, to accelerate workflow development. Ginkgo can now test tens of thousands of cells in parallel to identify the best cell. In addition, the system is linked to Ginkgo’s bioreactors, enabling the company to set its production at the single-cell level.
No more minipreps?
During the meeting, OriCiro Genomics announced its first product—the Cell-Free Cloning System. This product, the company declared, could replace conventional molecular cloning techniques based on small-scale plasmid preparation (miniprep) from Escherichia coli cultures. That’s right, OriCiro’s technology could eliminate minipreps!
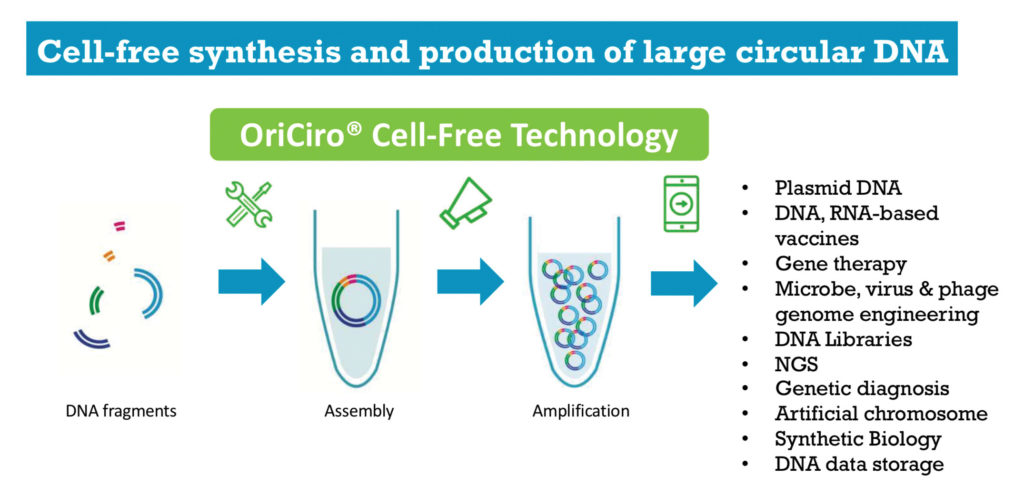
OriCiro had its origins in a Rikkyo University laboratory led by Masayuki Su’etsugu, PhD. After Su’etsugu devised the technology to assemble and amplify large, circular DNA, he co-founded OriCiro. Now, almost two years later, he is the CSO of the company, which employs a team of 10 and is located on the campus of the University of Tokyo.
By identifying the 26 proteins that are responsible for the E. coli amplification processes, Su’etsugu and the team have developed a kit with purified proteins that reconstitutes the process in vitro without the use of E. coli cells. Seiji Hirasaki, CEO and co-founder of OriCiro, tells GEN that the company’s technology accomplishes both assembly and amplification.
In the Cell-Free Cloning System, assembly progresses using homologous, overlapping ends, rather like Gibson assembly, to connect as many as 50 DNA fragments. Gibson assembly, first developed in 2009, is a widely used method for the assembly of multiple DNA fragments using one isothermal reaction, in one tube, without the need for restriction enzyme sites. One distinction is that the OriCiro kit uses an enzymatic annealing approach instead of the thermal annealing approach used by Gibson.
To accomplish amplification, the OriCiro system needs to prepare circular DNA that incorporates an oriC sequence. This is a DNA sequence that acts as the starting point of replication in E. coli.
The OriCiro kit starts at $500 (including 10 amplification and 5 assembly reactions), on top of the cost of the DNA. So, it is hard to envision minipreps becoming obsolete any time soon. Standard miniprep kits run about $1–2/prep.
Cultivating farming’s future
Over the past decade, the agribusiness has encountered increasing resistance to “processed foods,” observed D.A. Wallach, a singer and investor, during a talk with George Church, PhD, professor of genetics at Harvard Medical School, and Kira Peikoff, editor in chief at LeapsMag. The resistance, Wallach explained, was created by the notion that food is processed for the benefit of companies, not consumers. It’s a false choice, he asserted, to think that the only alternative to processing is to go back to “natural.” He added that the idea of a future of food heavily engineered for consumer preferences and human benefit is really exciting—a sentiment shared throughout the meeting.

Another panel at the event whet attendees’ appetites for a future with synthetic food. This panel, which was led by Christine Gould, founder and CEO of Thought for Food, included Lisa Dyson, PhD, CEO of Air Protein, and Michael Selden, CEO of Finless Foods. Selden explained that the maximum amount of fish that can be retrieved from the world’s oceans is already being taken each year. Seafood, he insisted, is very supply constrained. One way to alleviate pressure on the world’s seafood stocks is to create an alternative supply. Fish farming was an earlier proposed solution, but many species cannot be farmed. So, Finless is using animal cell culture to produce something that is, on a cellular level, “the same thing that people are eating today, just produced outside of a fish.”

Selden notes that salmon is some of the most efficient fish farming available, but it takes two years for a fish to get up to harvest weight. In contrast, Finless’ bioreactors turn around every week, providing constant production. In addition, they can provide food that is free of mercury and plastic contamination. The goal is to create food that is sustainable and ethical, and that people want to buy—not necessarily for virtue’s sake, but because the food is affordable, nutritious, convenient, and tastes good. The company wants to “shift the way that the entire world eats,” not just shift the ethical consumerism market.

Finless started in 2017 as one of the first cell-based seafood companies and the third cell culture meat company of any variety. Now, there are more than 50 worldwide. Gould noted that 10 years ago, people would say that making synbio food companies would be too hard because “consumers will never accept it and regulation will be too complicated.” But the “explosion of companies” over the past five years is changing the game.
Dyson’s company is introducing a new category of alternative meat production using molecules in the air—oxygen and carbon dioxide—as core building blocks in a process similar to fermentation. Air Protein makes a protein flour that is dense with nutrients such as essential amino acids, minerals, and vitamins. Then the company turns the flour into meat analogues—including beef, pork, poultry, and seafood—by using pressure, temperature, and natural flavors.
Dyson, who was named this year’s Bioinnovator of the Year at SynBioBeta, noted that she considers herself a farmer because Air Protein is innovating a process that is currently used in the food production industry. Gould adds that the synbio community is “shaking up this age-old industry and what it means to be a food producer,” especially given that worldwide the average farmer is more than 65 years old.
Feeding the world’s growing population despite resource depletion and climate change is just one global challenge that is being addressed by the synbio community. Synbio is also grappling with healthcare demands and the need for sustainable manufacturing. As SynBioBeta 2020 made clear, the synbio community has a lot on its plate.







