Researchers from the Salk Institute and the University of Sheffield completed a study that they say shows promise for the development of gene therapies to repair hearing loss. In developed countries, about 80 percent of deafness cases that occur before a child learns to speak are due to genetic factors.
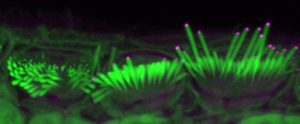
One of these genetic components leads to the absence of the protein EPS8, which coincides with improper development of sensory hair cells in the inner ear. These cells normally have long hair-like structures, called stereocilia, that transduce sound into electrical signals that can be perceived by the brain. In the absence of EPS8, the stereocilia are too short to function, leading to deafness.
The team’s findings “AAV-mediated rescue of Eps8 expression in vivo restores hair-cell function in a mouse model of recessive deafness,” published in Molecular Therapy—Methods & Clinical Development, indicate that delivery of normal EPS8 can rescue stereocilia elongation and the function of auditory hair cells in the ears of mice affected by the loss of EPS8.
“The transduction of acoustic information by hair cells depends upon mechanosensitive stereociliary bundles that project from their apical surface. Mutations or absence of the stereociliary protein EPS8 cause deafness in humans and mice, respectively. Eps8 knockout mice (Eps8−/−) have hair cells with immature stereocilia and fail to become sensory receptors,” write the investigators.
“The number of hair cells undergoing full recovery was not sufficient to rescue hearing in Eps8−/− mice. Adeno-associated virus (AAV)-transduction of P3 apical-coil and P1–P2 basal-coil hair cells does not rescue hair cells, nor does Anc80L65-Eps8 delivery in adult Eps8−/− mice.
“We propose that AAV-induced gene-base therapy is an efficient strategy to recover the complex hair-cell defects in Eps8−/− mice. However, this therapeutic approach may need to be performed in utero since, at postnatal ages, Eps8−/− hair cells appear to have matured or accumulated damage beyond the point of repair.”
Hair-cell function can be restored in certain cells
“Our discovery shows that hair-cell function can be restored in certain cells,” says co-senior author Uri Manor, PhD, assistant research professor and director of the Waitt Advanced Biophotonics Core at Salk. “I was born with severe to profound hearing loss and feel it would be a wonderful gift to be able to provide people with the option to have hearing.”
The cochlea, a spiral tube structure in the inner ear, enables us to hear and distinguish different sound frequencies. Low-frequency regions of the cochlea have longer stereocilia while high-frequency regions have shorter stereocilia. When sound travels through the ear, fluid in the cochlea vibrates, causing the hair-cell stereocilia to vibrate. These hair cells send signals to neurons, which pass on information about the sounds to the brain.
Manor previously discovered that the EPS8 protein is essential for normal hearing function because it regulates the length of hair-cell stereocilia. Without EPS8, the hairs are short. Concurrently, co-senior author Walter Marcotti, PhD, professor at the University of Sheffield, discovered that in the absence of EPS8 the hair cells also do not develop properly.
For this study, Manor and Marcotti joined forces to see if adding EPS8 to stereocilia hair cells could restore their function to improve hearing in mice. Using a virus to help deliver the protein to hair cells, the team introduced EPS8 into the inner ears of deaf mice who lacked EPS8. They then used detailed imaging to characterize and measure the hair-cell stereocilia.
The team found that EPS8 increased the length of the stereocilia and restored hair- cell function in low-frequency cells. They also found that after a certain age, the cells seemed to lose their ability to be rescued by this gene therapy.
“EPS8 is a protein with many different functions, and we still have a lot more to uncover about it,” notes Manor. “I am committed to continuing to study hearing loss and am optimistic that our work can help lead to gene therapies that restore hearing.”
Future research will include looking at how well EPS8 gene therapy might work to restore hearing during different developmental stages, and whether it might be possible to lengthen the therapeutic window of opportunity.