Staphylococcus aureus (SA) is an extremely common bacterial infection and about 30% of people have the microorganism living in their noses. However, SA can also be a cause of serious hospital-acquired and community-associated infections. And while a vaccine for the bacterium would be a game-changer for public health, for decades all vaccine candidates for SA have failed in clinical trials, despite being successful in preclinical studies in mice.
A study by researchers at the University of California (UC) San Diego School of Medicine may now have discovered why. Testing a new hypothesis, the team found that when SA first colonizes or infects humans, the bacteria can trick the body into releasing non-protective antibodies. When the individual is later vaccinated, these non-protective antibodies are preferentially recalled, making the vaccine ineffective.
“Somehow, SA is able to trick our immune system, and figuring out how will help us improve existing SA vaccines and develop new ones,” said George Liu MD, PhD, professor in the Department of Pediatrics at UC San Diego School of Medicine. “More broadly, these findings suggest a whole new way of reevaluating failed vaccines, which could have implications well beyond this one bacterium.”
Liu is senior author of the team’s published paper in Cell Reports Medicine, titled, “The characteristics of pre-existing humoral imprint determine efficacy of S. aureus vaccines and support alternative vaccine approaches.”
SA has a unique relationship with humans. The bacterium can exist in people as a normal part of the healthy human microbiome, living peacefully in the nose and on the skin. But SA can also cause many dangerous health complications, including wound and bloodstream infections. “As a successful human colonizer, Staphylococcus aureus (SA) balances a lifestyle of a symbiont and occasional deadly pathogen,” the authors wrote, citing CDC figures estimating that methicillin resistant SA strains cause an estimated 323,000 hospitalizations, and more than 10,000 deaths, annually. “SA’s role as a pathogen has earned it the categorical designation of “serious threat” to public health.”
Developing an effective SA vaccine has been a major goal of U.S. and global health organizations for the past decades, the team noted. But while While SA vaccine candidates may do well in preclinical studies in mice, they’ve unilaterally failed in clinical trials. “ … none of the approximately 14 Phase II and III trials have succeeded in meeting their respective target endpoints,” the authors commented. “The reason behind vaccine efficacy differences between humans and animal has remained unclear.”
The immune system releases protective antibodies in response to foreign antigens. These antibodies are then saved in the immune system’s memory, so the next time the immune system encounters that same antigen it will generally recall its earlier immune response rather than mount a brand-new attack.
“This is an effective system for conferring long-term protection against pathogens, but it only works when the initial immune response to that pathogen was actually protective,” said co-lead author JR Caldera, PhD, who completed his doctoral research in the Liu Lab. “What sets SA apart is that the bacteria themselves have ways of evading the immune system from the moment they encounter us, and these evasive strategies are only reinforced by vaccination.”
To investigate this further the researchers collected blood sera from nine healthy volunteers, quantifying and purifying SA-antigen-specific IgG antibodies present in the samples. They then transferred these antibodies to mice to explore how protective they were against SA on their own, as well as how they influenced the efficacy of several clinically tested SA vaccine candidates.
The researchers found that the vaccines were ineffective in mice that had been given human anti-SA antibodies, as well as in mice that had been previously exposed to SA. However, in mice that had never been exposed to either SA or human antibodies, the vaccines worked.
Unlike previous mouse studies of SA vaccines, the researchers’ results were consistent with those of failed clinical trials, suggesting that their experimental model could help predict the clinical success of SA vaccines while they are still being tested in preclinical mouse studies. “The universal failure in staphylococcal vaccinology has been a long-standing conundrum that we propose is rooted in flawed modeling and design,” they pointed out.
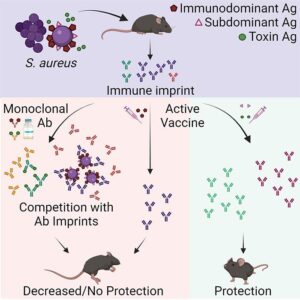
“One pathogen can have many different antigens that the immune system responds to, but there is a hierarchy as far as which antigen is dominant,” said co-lead author Chih Ming Tsai, PhD, a project scientist in the Liu Lab. “Most vaccines are based on the dominant antigen to trigger the strongest possible immune response. But our findings suggest that for SA, the rules are different, and it is more beneficial to target so-called subdominant antigens, which triggered a weak immune response in the first place.”
Liu added, “SA has been with humans a long time, so it’s learned how to be part-time symbiont, part-time deadly pathogen. If we’re going to develop effective vaccines against SA, we need to understand and overcome the strategies it uses to maintain this lifestyle.” And as the authors further noted in their study, “Overall, our study points to a practical and predictive approach to evaluate and develop SA vaccines based on pre-existing humoral imprint characteristics.”

