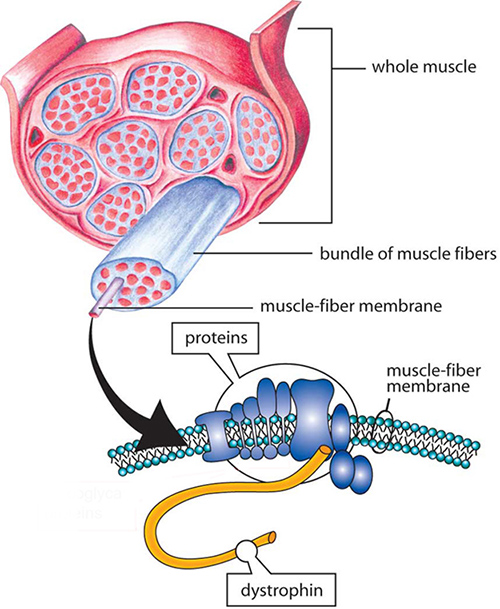
![Duchenne muscular dystrophy is caused by the inability to produce dystrophin, a long protein chain that binds the interior of a muscle fiber to its surrounding support structure. A new study may lead to clinical advances and help to eventually reverse these debilitating mutations. [Muscular Dystrophy Association]](https://genengnews.com/wp-content/uploads/2018/08/BMDDMD_musclecell8233245123-1.jpg)
Duchenne muscular dystrophy is caused by the inability to produce dystrophin, a long protein chain that binds the interior of a muscle fiber to its surrounding support structure. A new study may lead to clinical advances and help to eventually reverse these debilitating mutations. [Muscular Dystrophy Association]
The year 2015 has been a banner one for the field of genome editing. Molecular manipulation tools like the CRISPR/Cas9 system are becoming more refined and utilized to rectify a multitude of diseases in the laboratory, with an ever-watchful eye toward use in clinical trials.
In a landmark study, a research team led by scientists at Duke University has used CRISPR to treat an adult mouse model of Duchenne muscular dystrophy (DMD)—marking the first instance that CRISPR has successfully treated a genetic disease inside a fully developed living mammal—with a strategy that has the potential to be translated into human therapy.
The investigators had previously used CRISPR to correct mutations associated with DMD in vitro. However, these techniques were performed only on single-cell mammalian embryos. Earlier this year, the scientific community overwhelmingly opted for a moratorium to be placed on CRISPR research in connection with human embryos, so the Duke team was forced to find a new approach for treating animal models of DMD.
In the new study, the researchers used a nonpathogenic carrier called adeno-associated virus, or AAV, to deliver the gene-editing system. In a recent publication, scientists used this same virus to treat DMD in dogs, albeit using a gene therapy approach. The researchers were hopeful that their new method would overcome some of the editing efficiency issues seen in previous studies.
“Recent discussion about using CRISPR to correct genetic mutations in human embryos has rightfully generated considerable concern regarding the ethical implications of such an approach,” explained senior study author Charles Gersbach, Ph.D., associate professor of biomedical engineering at Duke University. “But using CRISPR to correct genetic mutations in the affected tissues of sick patients is not under debate. These studies show a path where that’s possible, but there’s still a considerable amount of work to do.”
The findings from this study were released today in Science through an article entitled “In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy.”
DMD is caused by the inability to produce dystrophin, a long protein chain that binds the interior of a muscle fiber to its surrounding support structure. Dystrophin is coded by a gene containing 79 protein-coding regions, called exons. If a single exon gets a debilitating mutation, the protein chain is malformed and ineffective—causing muscles to shred and slowly deteriorate.
Work on potential genetic treatments for DMD has been ongoing for many years. However, most traditional laboratory techniques do not represent a practical strategy for delivering CRISPR to a patient’s muscle tissue.
“A major hurdle for gene editing is delivery. We know what genes need to be fixed for certain diseases, but getting the gene editing tools where they need to go is a huge challenge,” noted lead author Chris Nelson, Ph.D., postdoctoral fellow in Dr. Gersbach’s laboratory. “The best way we have to do it right now is to take advantage of viruses because they have spent billions of years evolving to figure out how to get their own viral genes into cells.”
While early virus types didn’t work well for various reasons, such as integrating into the genome and causing problems or triggering immune responses, AAV thus far has proven special. Many people are exposed to AAV, and it is nonpathogenic, yet still exceptionally effective at getting into cells. Moreover, AAV is in use in many late-stage clinical trials in the United States and has already been approved for use in one gene therapy drug in the European Union.
However, there is a drawback. “AAV is a really small virus, and CRISPR is relatively large,” said Dr. Gersbach. “It simply doesn’t fit well, so we still had a packaging problem.”
The researchers decided to use the smaller Cas9 protein from Staphylococcus aureus, which they found was small enough to fit inside AAV, rather than larger and traditional Streptococcus pyogenes enzyme.
The Duke team first delivered the therapy directly to a leg muscle in an adult DMD mouse resulting in the restoration of functional dystrophin and an increase in muscle strength. Subsequently, they injected the CRISPR/AAV combination into a mouse’s bloodstream to reach every muscle. The results showed some correction of muscles throughout the body, including in the heart—a major achievement as heart failure is often the cause of death for Duchenne patients.
“There is still a significant amount of work to do to translate this to a human therapy and demonstrate safety,” concluded Dr. Gersbach. “But these results coming from our first experiments are very exciting. From here, we’ll be optimizing the delivery system, evaluating the approach in more severe models of DMD, and assessing efficiency and safety in larger animals with the eventual goal of getting into clinical trials.”