A radically redesigned viral vector may improve the delivery of therapeutic genes to patients with sickle-cell disease. According to tests in animal models, the new vector is up to ten times more efficient at incorporating corrective genes into bone marrow stem cells than conventional vectors, and it has a carrying capacity that is up to six times higher.
The conventional vectors that have been used to deliver gene therapies for sickle-cell disease have a reverse structural orientation. This orientation, which obliges the viral vector-making machinery to read the therapeutic genes in reverse, results in viral titers and transduction efficiencies lower than those that could be achieved with a forward orientation. For decades, the drawbacks of the reverse orientation were accepted because it was a convenient way to prevent a persistent problem attributed to RNA splicing.
Basically, treating sickle-cell disease with gene therapy requires that the delivery vector’s various components include intron 2. It is required for high-level expression of another key component, the β-globin gene. Intron 2, however, gets “clipped out” during the normal vector preparation process if it is left in the natural, forward direction.
To move the delivery of the β-globin gene forward—in both senses, researchers based at the National Institutes of Health (NIH) have been working on a unique workaround. John Tisdale, MD, chief of the Cellular and Molecular Therapeutic Branch at the National Heart, Lung, and Blood Institute (NHLBI), has led an effort to create a viral vector that leaves intron 2 intact but also incorporates a new forward-oriented β-globin vector.
In contrast to the old vector, the gene sequence, or “message,” of the new β-globin vector is read forward, making the gene translation approach less complicated.
“Our new vector is an important breakthrough in the field of gene therapy for sickle cell disease,” said Tisdale. “It’s the new kid on the block and represents a substantial improvement in our ability to produce high-capacity, high-efficiency vectors for treating this devastating disorder.”
Details about the latest work on the new vector appeared October 2 in Nature Communications, in an article titled, “Development of a forward-oriented therapeutic lentiviral vector for hemoglobin disorders.” The article indicates that the new vector is easier and cheaper to manufacture.
“Here we report a clinically relevant forward-oriented β-globin-expressing vector, which has sixfold higher vector titers and four- to tenfold higher transduction efficiency for long-term hematopoietic repopulating cells in humanized mice and rhesus macaques,” the article’s authors wrote. “Insertion of Rev response element (RRE) allows intron 2 to be retained, and β-globin production is observed in transplanted macaques and human SCD CD34+ cells.”
The new vectors also showed a capacity for longevity, remaining in place four years after transplantation. Researchers also found that they could be produced in much higher amounts than the conventional vectors, potentially saving time and lowering costs associated with large-scale vector production.
Gene therapy trials using reverse-oriented vectors for sickle-cell disease and β-thalassemia have largely been encouraging. But better results, the researchers suggested, might be attainable by optimizing target cell collection, processing, and transduction: “Indeed, lower than anticipated gene transfer levels in preliminary gene therapy trials are likely the result of reduced vector copy number, as reverse-oriented globin vectors have lower vector titers and lower transduction efficiency in primary human hematopoietic stem cells, which limits their clinical prospects. We hypothesized that reverse orientation impedes both viral preparation and vector transduction.”
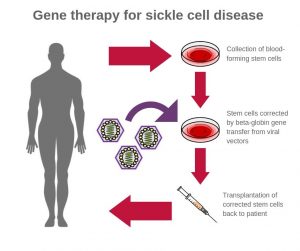
“Our lab has been working on improving β-globin vectors for almost a decade…and finally decided to try something radically different—and it worked,” Tisdale said. “These findings bring us closer to a curative gene therapy approach for hemoglobin disorders.”


