 Fyodor Urnov, PhD, is a pioneer in the field of genome editing and one of the scientists most invested in expanding the availability and utility of CRISPR-based therapies to the broadest possible population. He envisions a world in which genome editing can treat the nearly 400 million people who are suffering from one of the 7000 diseases brought on by gene mutations.
Fyodor Urnov, PhD, is a pioneer in the field of genome editing and one of the scientists most invested in expanding the availability and utility of CRISPR-based therapies to the broadest possible population. He envisions a world in which genome editing can treat the nearly 400 million people who are suffering from one of the 7000 diseases brought on by gene mutations.
After his PhD in 1996 from Brown University, Urnov worked as a postdoctoral fellow in the laboratory of Alan Wolffe at the National Institutes of Health (NIH). In 2000, Urnov joined Wolffe in moving to Sangamo Therapeutics in California. During his 16 years at Sangamo, Urnov and his colleagues performed the first demonstration using zinc-finger nucleases to modify DNA in human cells in 2005, coining the term “genome editing” in the process.1
After that, Urnov led collaborative teams that created large-scale genome editing applications in crop genetics, model animal reverse genetics, and human somatic cell genetics. While at Sangamo, Urnov also led a cross-functional team from basic discovery to the initial design of the first-in-human clinical trials for sickle cell disease and beta-thalassemia, which are being conducted in collaboration with UCSF Benioff Children’s Hospital and UCLA Broad Stem Cell Research Center.
In 2019, Urnov became the director of the Center for Translational Genomics at the Innovative Genomics Institute (IGI), working alongside Nobel laureate Jennifer Doudna, and a professor in the Departments of Genetics, Genomics, and Development at the University of California, Berkeley. At the IGI, Urnov works in collaborative teams to develop first-in-human applications of experimental CRISPR-based therapeutics for sickle cell disease (with Mark Walters, UCSF), genetic disorders of the immune system (with Alexander Marson, UCSF/IGI), radiation injury (with Jonathan Weissman, MIT/Whitehead Institute), cystic fibrosis (with Ross Wilson, IGI), and neurological disorders (with Weill Neurohub and Roche/Genentech).
In this exclusive interview, GEN Biotechnology talks to Urnov about his career in genome editing, from his early days at Sangamo to the establishment of his current company, Tune Therapeutics, which he cofounded with Charles Gersbach and Akira Matsuno (president and CFO). He elaborates on his plans for “CRISPR cures on demand” and the challenges that stand in the way of his goal.
(This Close to the Edge video interview below has been lightly edited for length and accuracy.)
Jonathan Grinstein: I read through your 2021 article for Molecular Therapy (“Imagine CRISPR Cures”),2 which I am guessing is a reference to the John Lennon song, and your 2022 op-ed for the New York Times (“We Can Cure Disease by Editing a Person’s DNA. Why Aren’t We?”).3 In those articles, you lay out the improvements necessary to make CRISPR cures for n = 1 diseases and rare diseases a reality. Where are we today in realizing your CRISPR-cure-on-demand vision?
Urnov: We have in front of us clinical data that genetic therapies for severe disease can be curative. This wasn’t a given. Genetic engineering to treat disease was proposed in 1972 by Ted Friedman at UCSD. That’s 50 years ago! The first gene therapy trials were done at the NIH in 1989. The first glimmers that gene therapy can work came in the 2000s; CRISPR came online in 2012; the first human was treated with CRISPR in 2019. Looking back at that time, it staggers the imagination how this early period of incubation—1989 through the early 2010s—where things were sort of working, sometimes there are glitches. But then the field hit its stride and we now have on the order of 15–20 gene therapies just for disorders of the blood alone, where we have pretty spectacular curative effects.
And when I say curative, I don’t mean a patient gets mildly better. I mean something like adenosine deaminase deficiency, severe combined immune deficiency. Don Kohn (UCLA) and Claire Booth (University College London) had 50 children who were certain to die, and they are basically cured by gene therapy or in two cases by bone marrow transplant. Think about that!
Similarly, as one looks at what CRISPR has been doing clinically, look at the data from clinical trials for sickle cell disease from CRISPR Therapeutics and Vertex Pharmaceuticals. They have treated people who have had multiple episodes of pain before being administered their own CRISPR-edited cells. And they have shown that dozens of human beings are now free of pain episodes (in the case of sickle) or need for transfusion. Or look at Intellia, which is treating ATTR amyloidosis; within a month of being administered a teaspoon of CRISPR—it is astonishing. You have 95% reduction in the bloodstream of these human beings of this toxic protein. So, blood is editable, the liver is editable. Major companies, biotech and pharma, are showing how well it works.
Nobody is celebrating this in the rare disease space because the rare diseases under the current system are just going to be left by the roadside. There are just not enough human beings with, say, rare disease number 75 out of 5000 to justify the commercial investment in taking that medicine through development, clinical trials then regulatory approval… We have examples where companies took on genetic therapies that they simply could not figure out how to commercialize… There are 17 diseases where lentiviral gene therapy was curative, the list is growing—but only four of them are commercialized.
For about three of the others, commercialization has been halted and none of the recent ones are being commercialized. So as Kohn says, the list of diseases we have cured is growing at the bottom and the list of diseases that are commercialized and approved is shrinking from the top.
The realization that CRISPR can be this powerful is now a definitive component of the momentum that the system has to change. I am unaware, unfortunately, of a single gene-editing trial anywhere in the world for genetic disease that will be all academic and all nonprofit—other than the one we have, which is led by Mark Walters (University of California, San Francisco). It gives me zero pleasure to say that we are the only ones. There should be literally 100 trials such as this—the patients are out there and the technology is there. So, as we think about getting closer to a world where these diseases are not left by the wayside, we are probably 20% of the way in. I will also say that the remaining 80% are going to be more challenging than the first 20%.
The first 20% of the way that we are in basically shows that academic and nonprofit centers have all the relevant expertise to design a CRISPR medicine, to administer it to animals, and in a few cases really get the program to a point where, if only we could manufacture the medicine affordably, if only we could go through clinical trials affordably, if only there was a regulatory framework where we would not be burdened by studies that are millions of dollars and years in length, which is what currently the costs are.
The next three to five years, I see as almost a moral must for our field. We have to take the momentum that CRISPR can be curative, that gene therapy can be curative. We have to take the established fact that there are academic and nonprofit institutions across the world with CRISPR expertise. I am sitting in the center of one of them (UC Berkeley); our sister campus at UCSF is a world leader in developing these therapies.
Our sister campus, UCLA, is another leader. We at UC Berkeley are a CRISPR center of excellence. There are other places like that, Children’s Hospital Boston, Penn Medicine, Seattle Children’s, St. Jude. What currently does not exist is a way to support these institutions and create a dedicated manufacturing and regulatory framework for them where, within the realm of academic nonprofit medicine, they can start rapidly developing, de-risking and administering these cures for n = 1.
Why don’t we have that? Well, we have never had a technology as versatile as CRISPR. In other words, the reason that there is not some sort of wonderful environment where you can develop and deliver a CRISPR cure in 6 months is we have never had a reason to build it! Now we do. The momentum to have the regulatory manufacturing and logistical and clinical environments now aligned with the promise of the technology comes from the fact that the technology has demonstrated definitive curative potential.
Grinstein: You are the cofounder of a biotech company called Tune Therapeutics. Can you give us the Spark Notes summary of epigenome editing? What can epigenetic editing do that base and prime editing cannot? If it is not a one-and-done treatment, why would you go this route as opposed to using a genetic tool like CRISPR for permanent changes?
Urnov: I really hope you booked three hours for this interview!
Why does fiber protect from colon cancer? It is because, in your colon, the fiber gets fermented to make a chemical called sodium butyrate, which enters the cells of the lining of the colon, and it changes chemical marks on genes that protect those colon cells from cancer. Those marks on the protein coating of the genes and on the DNA, itself don’t change what the genes say, those marks change what the genes do.
So next time you have some oatmeal, close your eyes, and visualize that fiber being fermented in your colon, making butyrate and the butyrate entering the cells that line your colon entering the nucleus and that chemical keeping the genes that protect you from cancer on. First of all, fiber is good for you, both for cardiovascular disease and colorectal health. This is a great example of how our genes learned from experience because that is literally what epigenetics is. Yes, we leave this mortal coil with more or less the same DNA that we are born with, but what our genes do in our lifetime changes, not just because we age, but because we go through various exposures. We know from striking public health evidence how powerful keeping a healthy epigenome can be.
In the United States and many developed countries, if a woman chooses to become pregnant, her physician will recommend that she takes a dietary supplement called folate. Folate is less interesting than what it does. It allows the developing fetus to have a healthy epigenome specifically in its spine. It prevents the prevalence of spina bifida. And there is definitive epidemiological evidence that dietary folate in a woman who chooses to have a biological child before conception and through pregnancy will keep the genes in the developing fetus and the baby that contribute to normal spine development in a healthy state…
So, the epigenome is the sum total of these little marks from experience that our genes acquire as we go through development and life. We have known about this since the 1960s, we have known that our DNA is enveloped in proteins that have these chemical marks, and those marks have something to do with what the genes do.
But until about 20 years ago, we were like astronomers staring at the stars, right? We can count them, but we cannot fly to them. Then about 20 years ago, some really amazing work that came out of a number of academic institutions and then ultimately got taken up by a technology company [Sangamo] where I have to disclose an emotional conflict of interest—I worked there for a decade and a half! You can engineer proteins that will recognize a specific gene inside a living cell and change its epigenome. What does that mean in practical terms? Imagine a gene that apparently got silenced for some reason of environmental exposure. Maybe we can wake it. Imagine a gene that is producing something unwanted; maybe we can build a protein that would engage that gene and turn it off.
We don’t have to imagine—this is all reality. Studies for the past 20 years have given us a proof of concept that you can turn genes on and off on demand. We need not wait for Mother Nature to smile benevolently on us, we can turn genes on and off on demand by building these epigenome editors. Notice the epi prefix: they are not gene editors, they don’t change the DNA; they change what the genes do. An epigenome editor can go inside a T cell or a brain cell and turn a specific gene on and off. I want to emphasize that we humans have a great ability of proposing technologies and then getting it to work as a proof of concept, and then other technologies come on board and ultimately, it is the constellation of things that makes things real.
My favorite example is the surface of the iPhone, made out of something called gorilla glass, which Corning engineered in the 1960s for use in windshields. It never caught on and sat on the shelf until Steve Jobs decided that he wanted to make his iPhone with an unbreakable glass cover. It is a great example of how multiple threads of technology come together to have a 1 + 1 = 7 effect! I think this is true for epigenome editing. We have known that we could do this since the early 2000s, but as you think about the ability to engineer new kind of proteins, both in terms of engaging the DNA and changing the epigenome, as we think about ways to rapidly profile their potency, do they do what we need them to do and how specific are they? Do they go somewhere else and turn some other genome?
As we think about ways to deliver them to specific cells or organs in the body, all of that has not just undergone an incremental change in the past 20 years it has undergone a step change, where we can take a large animal, like a human primate, and inject it with a teaspoon of an epigenome editor formulated with a lipid nanoparticle (LNP). You inject it into the circulation of a monkey. And inside that LNP is an epigenome editor, engineered to turn off a gene that contributes to cardiovascular disease. Lo and behold, within a couple of weeks of administering this epi editor, the gene goes off.
This is an amazing achievement, and the gene stays off for as long as the system has been looked at. We have always wanted to be able to tweak genes on and off—not just all the way on or off, but think of it as a soundboard, a bit more bass, a touch less treble, a bit more on the drums, and certainly less cowbell! This is what epigenome editing lets you do. It is like you can flip a gene on, you can flip a gene off, but you can also adjust it. Sound output… You don’t need to change the DNA sequences. You simply inscribe new molecular makeup on that gene without changing what the DNA says and the gene politely obliges. That is the Spark Notes version.
Grinstein: Is Tune Therapeutics the realization of a dream that started 25 years ago when you were a postdoc with the late Alan Wolffe?
Urnov: Absolutely! Alan believed in epigenetics and chromatin as being the key to real insight into how human genes work before most people did. At the time in the 1990s, we knew chromatin existed, but people thought that chromatin gets out of the way so that the real action can begin. We now know that is not the case, but at the time it was not front and center in the minds of people working on gene control or people building therapies. Alan was remarkably ahead of his time in two ways. First, he just thought about chromatin as just incredibly deep, even though we did not know how deep the rabbit hole goes. Second, he was young. He passed away tragically in an accident in 2001 (age 42).
His professional output over the previous 20 years was staggering. He was the youngest laboratory chief appointed to a chief of laboratory position at the NIH in its history. He wrote the definitive monograph on chromatin and epigenetics, which was on the table of everyone working in the field. And I will never forget the single most impactful conversation of my professional life, when Alan shows up at my bench in his laboratory in late 1999. Alan was English. He said, “Dr. Urnov, I have just had the most remarkable visit.” And he proceeded to describe the vision of engineering gene control using chromatin, epigenomic-based principles using a class of engineered proteins called zinc fingers.
As far as I was concerned, Alan was Yoda except much younger. I could not believe my luck that I got to work in his laboratory. He described this vision and said, “There’s a biotechnology company in Point Richmond, California.” I said, “That’s amazing, Alan, thanks for telling me.” Six weeks later he calls me into his office and says, “Dr. Urnov, I have a question for you. How committed are you to a career in academia?” I remember thinking, he does not think I have it in me to be an academic scientist. What have I done? But Alan was inviting me to join him at Sangamo.
A number of us went, it was the best professional move of my life. We had this extraordinary moment in 2001 where the field had just begun to realize what epigenetics could really do. And we had just begun to characterize these amazing molecular machines that inscribe epigenetic code. And to do the first experiments where we could bring in these epigenome modifiers, this was work by my colleague Philip Gregory, to endogenous human genes, and just instruct them by rewriting their epigenome.
Philip would show the data at Sangamo meetings. I remember thinking, where is this going to go? Then in 2002, there were some articles on fruit flies and frogs from Dana Carroll at the University of Utah, and an article from David Baltimore’s laboratory with Matt Porteus on targeted genetic engineering using the very same zinc fingers that we were using to change the epigenome. But then there was a severe adverse event in the gene therapy trial for bubble boy disease in France; 4 of 19 children developed cancer because the virus went into the wrong place [in the genome].
There we were, in 2002. We have zinc fingers, which can let us get to a gene of interest. We have early evidence from flies, frogs, and reporter genes in human cells that we can create a double-strand break and repair a mutation, and we have this unmet need. We pivoted: It is not that we did not think that epigenome editing was exciting, but in terms of fixing a mutation for bubble boy disease, that felt real, like we could do this. So, we went after that. Now the rest is history, right? We got genetic editing to work. We named the technology, did all the first clinical trials, and of course we are about to get our first approved editing medicine using CRISPR-Cas technology.
But all along, we were in Point Richmond, California, gene editing away. There was a hardy group of believers who never forgot that epigenome editing is a thing. And Charlie Gersbach (Duke University), my [Tune] cofounder, was one of those believers. He had his eye on that notion that you can inscribe epigenetic marks on genes, and he never took his eye off. I am very grateful to him. While everybody is running around making double-strand DNA breaks and creating interesting genetic forms, Charlie and some other academics thought this is all great, but we don’t have to change the DNA to change what the genes do (figure 1).
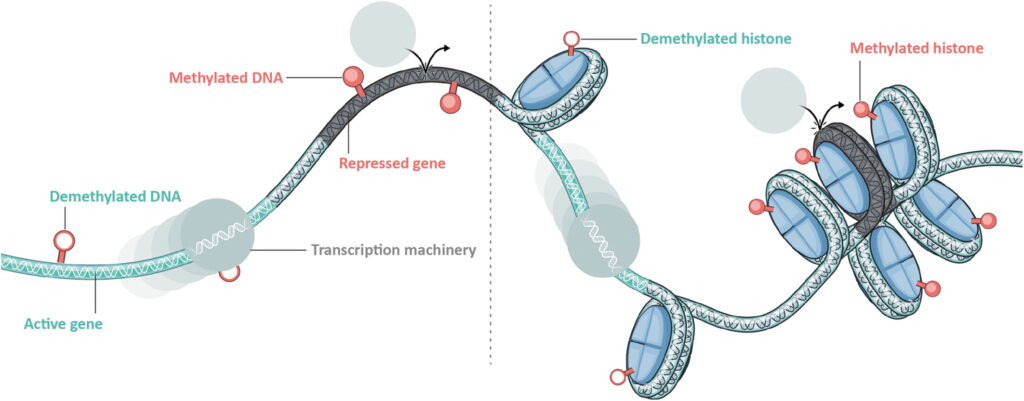
Epigenetic control elements are located along the length of each chromosome and alter local interactions between DNA and the histones. This keeps some genes coiled and inaccessible while opening up and making available others. These regulatory elements can alter chromatin structure by modifying histone proteins or DNA methylation and demethylation. This allows for the controlled activation or repression of genes across a wide range of cellular contexts and physiological states. [Tune Therapeutics]
About once a week, I see a scientific result where my first thought is, I wish Alan were alive to see this… When Tune showed the data that you can administer an epi editor to silence PCSK9 in the nonhuman primate with a durable effect, the first thought in my mind was, I wish Alan were alive to see this. I know exactly how he would have reacted. It is bittersweet.
Grinstein: What sets Tune apart from other epigenome editing companies?
Urnov: I love my field of targeted genetic and epigenetic engineering. We have a history of being a large rising tide that lifts all boats. It is a bit hard for me to say company #1 is better than company #2 because, for example, the people who have started at another epigenome editing company are some of my best professional friends. Rather, I would love to talk about what makes Tune strong, because as we have learned from a 30-year history of gene therapy, the more for-profit entities push technologies forward, the better we will get to learnings from clinical trials and preclinical development that gets us all to better platforms that become.
What makes Tune strong are three things. I spent 16 years in industry. The only thing that matters with respect to ultimate success for a therapeutic: you can have a lot of money, amazing technology, and tremendous unmet need. But if you don’t have the right people in the organization, it is going to fail. I think that the Tunesmiths, the melody makers, are some of the most impressive constellations of cross-functional expertise that I have ever seen.
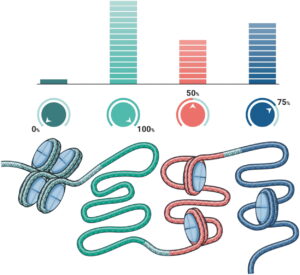
Genetic tuning, or epigenetic editing, involves tinkering with epigenetic machinery in order to alter the expression of genes. Rather than completely activating or silencing a gene, genetic tuning allows for a much more nuanced range of effects. In certain contexts, a partial reduction or activation of gene expression is preferable to total knockout or forced over-expression. These distinctions are important to circumvent many of the technical and clinical barriers that have hindered the development of previous genomic therapeutics. [Tune Therapeutics]
We have people who deeply understand how to engineer proteins, both in terms of routing them to specific positions in the DNA potently and specifically and in terms of what to fuse to them to create specific epi states. We have people with extraordinary skill in understanding how to read out at the cell biological level. Did we get the readout that is necessary? But all we have done so far is we have stayed in the same laboratory, right? We have built a protein that does something to a gene in a cell—that does not a therapeutic make (figure 2).
I am so impressed with the vigor and vision with which Tune’s leadership has been able to weave together a vertically integrated organization where pretty much at every station in the end-to-end journey of conceptualizing a target to then a disease therapeutic, and to then writing the target product profile, which is basically like, what are we treating? Using what? What is it going to do? What is the biological activity? What is the minimum approvable endpoint? What is the optimal problem from when you conceptualize target product profile for a disease indication too?
When you flesh out the paths of attack of how you are going to deploy your platform, what you are going to need, what does the preclinical package look like, to actually doing all the relevant manufacturing tasks, and then taking it through regulatory and having the clinical perspective. I think the cross-functional team that Tune has built out is what makes it strong.
Component number two is what these people have built. I am no stranger to impressive science, I have worked in organizations that know what they are doing. The Tune data are amazing. …We first met for a serious conversation about building Tune at a meeting. It was raining, so we were all glad to sit in the conference room in November in DC. And we sketched things out. I texted Charlie and said, well this has taken our dreams to reality and then some. I think the technology and the data that have come out of Tune—you feel parental, right? You teach your baby something and then you send them to college, and then you hold your breath and hope they write, but to have the report card come back and shine so brightly, that is strength number two.
Strength number three is the following: We have cured every mouse on earth of every disease known to humankind. The only way to learn how to treat disease is to treat people with a disease. No amount of preclinical efficacy and safety data can teach you the key things you need to know in terms of how to actually build a medicine for that disease. So, I think Tune’s strength is the clarity, vigor, and vision that leadership has managed to build and infuse the entire company with respect to a robust and healthy focus of getting Tune epi editors into the clinic.
I have seen biotechnology companies perhaps too enamored of their preclinical experimentation. You have to get to human beings. I salute our leadership—seasoned professionals with scars of what can go wrong in the clinic—for the way they have been able to configure the organization toward having a very healthy and vibrant R&D pipeline while time pushing the company in a healthy way toward getting us into the clinic.
References
1. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature 2005;435(7042):646–651; doi: 10.1038/nature03556 Crossref, Medline, Google Scholar
2. . Imagine CRISPR cures. Mol Ther 2021;29(11):3103–3106; doi: 10.1016/j.ymthe.2021.10.019 Crossref, Medline, Google Scholar
3. Urnov F. Opinion | We Can Cure Disease by Editing a Person’s DNA. Why Aren’t We? The New York Times; 2022. Google Scholar
To view this Close to the Edge video interview in its entirety click here.
This article was originally published in the October 2023 issue of GEN Biotechnology. GEN Biotechnology, published by Mary Ann Liebert, Inc., is the new, marquee peer-reviewed journal publishing outstanding original research and perspectives across all facets of the biotech industry.

