Despite their capacity to spread debilitating pathogens, research on ticks has lagged behind other arthropod vectors, such as mosquitoes. This is, in large part, because of challenges in applying available genetic and molecular tools. For example, up until now, successful gene editing had not been reported in ticks. Now, a team at the University of Nevada reports the first successful CRISPR-Cas9 genome editing in the black-legged tick. This milestone was made possible due to a novel embryo injection protocol developed by the researchers.

“Having genome-editing tools available will allow us to unlock some of the secrets of the tick genome and allow us to determine how these unique animals survive in the environment, how they interact with pathogens, and how we might prevent ticks from spreading diseases to humans and livestock,” said Monika Gulia-Nuss, PhD, associate professor in the department of biochemistry and molecular biology at the University of Nevada, Reno.
This work is published in iScience in the paper, “Cas9-mediated gene editing in the black-legged tick, Ixodes scapularis, by embryo injection and ReMOT Control.”
“Previously, no lab has demonstrated genome modification is possible in ticks. Some considered this too technically difficult to accomplish,” Gulia-Nuss said. “This is the first study to demonstrate that genetic transformation in ticks is possible by not only one, but two different methods.”
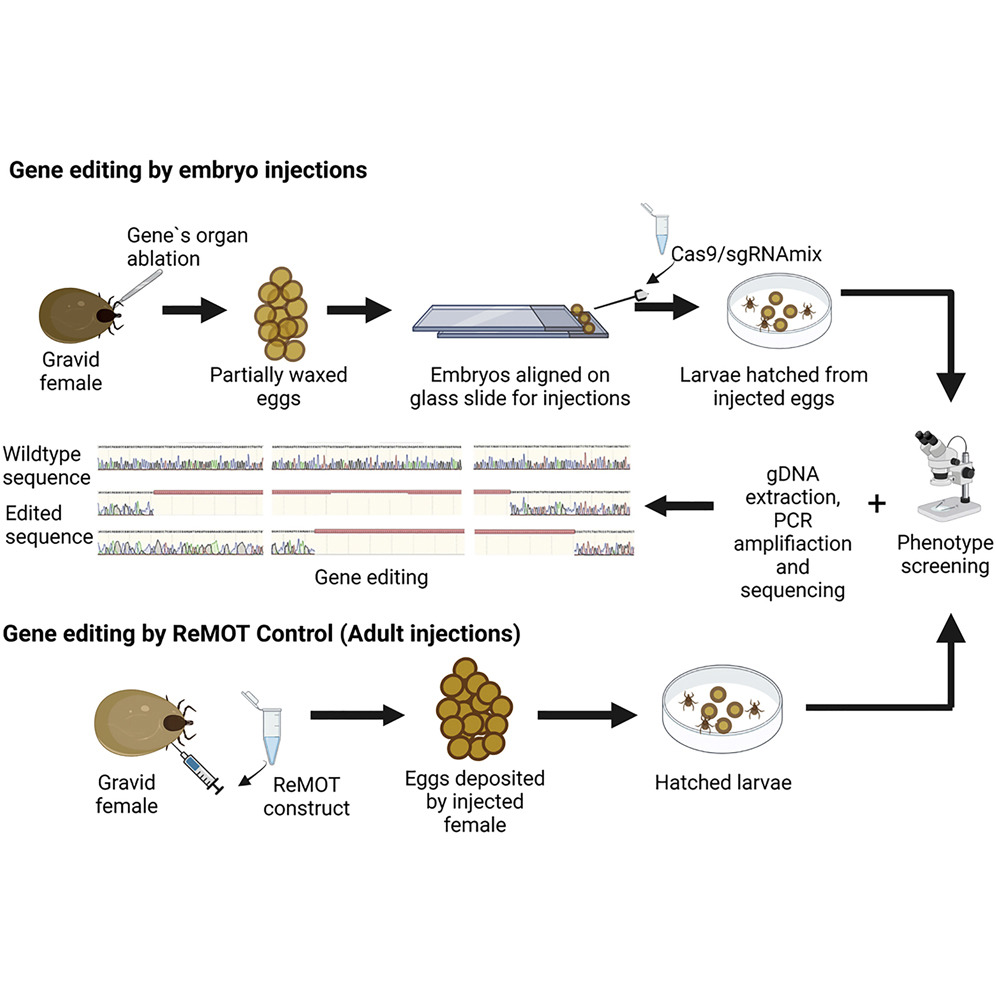
CRISPR-Cas9 has revolutionized functional genetics research in many organisms. This gene-editing technique has been applied to mosquitoes and other arthropod disease vectors, but successful gene editing has not yet been reported in ticks. Technical challenges for injecting tick embryos to attempt gene editing have further slowed research progress. Tick embryos are extremely difficult to inject because of high pressure inside the eggs, a hard chorion (the outer shell of the egg), and a wax layer outside the embryo that must be removed before injection. Female ticks use a specialized organ called Gene’s organ to coat their eggs with a tough wax layer.
Researchers developed a successful tick-embryo injection protocol and targeted gene disruption with CRISPR-Cas9 using two methods: embryo injection and Receptor-Mediated Ovary Transduction of Cargo (ReMOT Control)—a less labor-intensive method of gene editing in arthropods. The researchers ablated Gene’s organ to prevent wax deposition and then treated the eggs with chemicals called benzalkonium chloride and sodium chloride to remove the chorion and to decrease the pressure inside the eggs.

“We were able to carefully dissect gravid female ticks to surgically remove the organ responsible for coating the eggs with wax, but still allowing the females to lay viable eggs. These wax-free eggs permitted injection of tick embryos with materials necessary for genome modification,” Gulia-Nuss said. “Another major challenge was understanding the timing of tick embryo development. So little is known about tick embryology that we needed to determine the precise time when to introduce CRISPR-Cas9 to ensure the greatest chance of inducing genetic changes.”
The survival rate of injected embryos was approximately 10%, comparable to well-established insect models. For ReMOT Control, all injected ticks survived. The data showed the feasibility of tick embryo injection and genetic manipulation in ticks by both methods, which had comparable editing efficiency for the Proboscipedia (ProbP) gene, which is expressed in appendages.
More research is needed to fully understand the molecular mechanisms underlying efficient gene editing in ticks. While these tools will accelerate tick genetic research, improvements are needed in the embryo injection protocol to enhance survival and larval hatching and gene-editing efficiencies.
“We expect that the tools we developed here will open new research avenues that will dramatically accelerate our understanding of the molecular biology of this and related tick species,” Gulia-Nuss said. “Targeted disruption of genes in tick vectors of human pathogens is a powerful method to uncover the underlying biology of tick-pathogen-host interactions that can inform the development and application of new approaches to tick-borne disease control.”