Researchers describe a new method of studying the endocytosis and recycling of cell surface proteins.
ASSAY & Drug Development Technologies offers a unique combination of original research and reports on the techniques and tools being used in cutting-edge drug development. The journal includes a “Literature Search and Review” column that identifies published papers of note and discusses their importance. GEN presents one article that was analyzed in the “Literature Search and Review” column, a paper published in ACS Chemical Biology titled “Releasable SNAP-tag probes for studying endocytosis and recycling.” Authors of the paper are Cole NB and Donaldson JG.
Abstract from ACS Chemical Biology
Site-specific labeling of cellular proteins with chemical probes is a powerful tool for live cell imaging of biological processes. One popular system, known as the SNAP-tag, is based on an engineered variant of the 20-kDa DNA repair protein O6-alkylguanine- DNA-alkyltransferase (AGT) that covalently reacts with O6-benzylguanine (BG) and can be derivatized with a number of reporter groups. For studying the endocytosis and recycling of cell surface proteins, the covalent nature of BG binding to the SNAP-tag is problematic, since removing excess noninternalized probe from the cell surface is not feasible.
Here we describe a modification of the SNAP-tag technology that permits the rapid release of fluorescently labeled probes from the cell surface without affecting the population of labeled molecules sequestered within endosomes. This simple yet effective approach allows quantitative measurements of endocytosis and recycling in both imaging and biochemical assays and is especially useful when studying endosomal dynamics in live cells.
Commentary
Protein fusions to the DNA repair protein O6-alkylguanine-DNA-alkyltransferase (AGT), the so-called SNAP tag, have been used to construct a variety of assays. Assays for endocytosis have typically involved high-content imaging approaches using green fluorescent protein (GFP)-tagged membrane receptors. The work described here uses an O6-benzylguanine (BG) linked to a fluorophore via a disulfide bond.
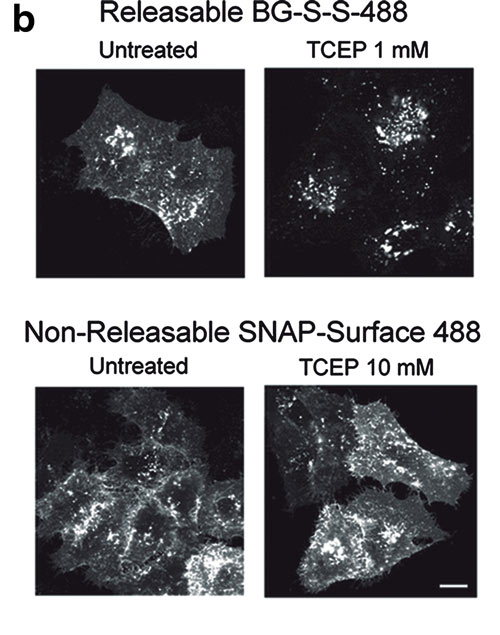
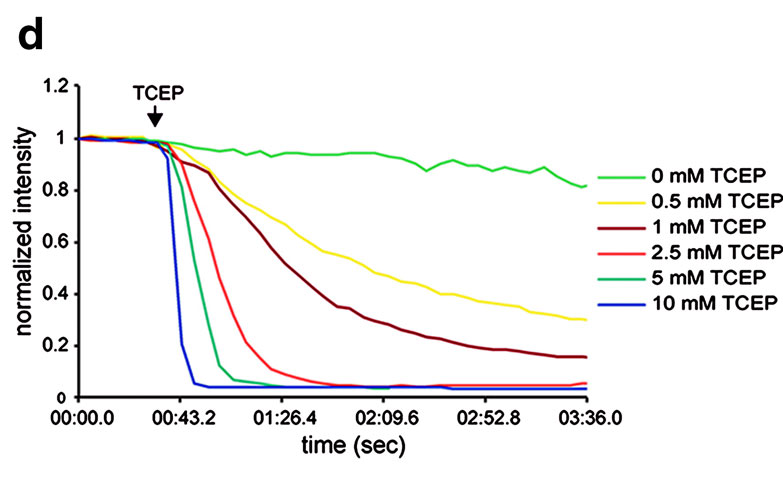
Labeling of a cell surface receptor fused to a SNAP-tag allows monitoring endocytosis by removing the surface bound fluorophore with a cell-impermeable reducing agent such as tris(2-carboxyethyl)phosphine (TCEP). Following TCEP treatment of cells, only the internalized fluorophore is monitored (see Figures A–D). The endosomal environment is thought to be generally nonreducing, and so the internalized BG-S-S-488 probe is not cleaved in the endosome.
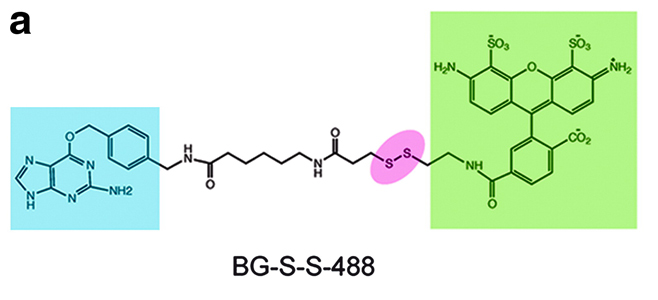
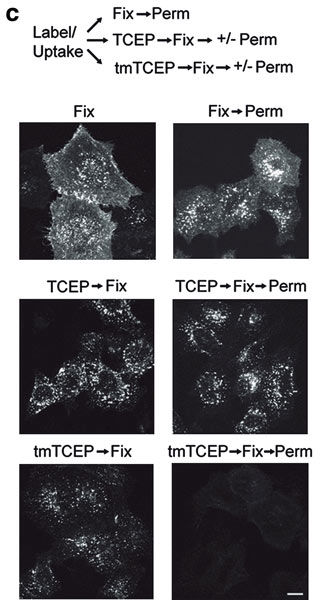
To test this, the authors used a cell permeable form of TCEP (a trimethyl ester analog, tmTCEP). No free dye could be extracted from cells containing endosomes with SNAP-β2-adrenergic (ADR) which were labeled with BG-S-S-488 when TCEP was used; however, free dye was extracted from these cells when tmTCEP was used. Therefore, the normal environment of endosomes is apparently nonreducing. Endocytosis and recycling can be quantified with this method because the same cells can be used to measure the total fluorescence from labeled receptors on the surface (before treatment with TCEP) as well as the internalized fluorescence after treatment with TCEP.
A two-color assay is also shown in this article in which an RFP-tagged β2-arrestin, and either SNAP-β2ADR or SNAP-NK1R are imaged. Agonist treatment shows translocation of the RFP-β2-arrestin2 to the cell membrane, and following TCEP treatment the co-localization of the receptors into endosomes could be measured. With this method different populations of receptor/β2-arrestin complexes can be studied.
The assay can be applied to any cell surface protein and could be expanded to study other internalization processes such as phagocytosis as well.
Doug Auld, Ph.D., is affiliated with the Novartis Institutes for BioMedical Research.

