The human genome encodes hundreds of G protein-coupled receptors (GPCRs), which form the largest group of receptors that hormones and neurotransmitters exert their functions on our cells. Because of this GPCRs are of extreme importance as drug targets, as roughly half of all prescribed drugs act on these receptors. Moreover, GPCR targeted therapies help in the treatment of widespread diseases such as hypertension, asthma, and Parkinson’s.
For many years, scientists were convinced that GPCRs sat at the cell surface and only from there could they influence the activity of the cell, via activation of various intracellular signaling cascades. New data from investigators at the Bio-Imaging Center at the University of Würzburg has shaken these long-standing beliefs, uncovering evidence suggesting that GPCRs are also active in the cell interior. Findings from the new study were published recently in Nature Communications through an article entitled “Internalized TSH receptors en route to the TGN induce local Gs-protein signaling and gene transcription.”
In simplified terms, G-protein-coupled receptors sit at the cell membrane waiting for a hormone or neurotransmitter to bind and thereby activate them. The signal is then transmitted inside the cell, mainly through the production of an intracellular second messenger such as cyclic adenosine monophosphate (short cAMP). This second messenger, in turn, is involved in the regulation of a large number of cell functions, such as gene transcription and cell division.
“The first indication that GPCRs also initiate the production of cAMP in the cell interior came from two studies of typical protein hormone receptors,” explained senior study investigator Davide Calebiro, Ph.D., a professor at the Institute of Pharmacology and Toxicology and the Bio-Imaging Center of the University of Würzburg. “Those studies independently showed that GPCRs can induce a second, persistent phase of cAMP production in the cell interior.”
Interestingly, this phenomenon was shown to be biologically relevant. However, the exact mechanism was largely unknown. Dr. Calebiro and his team were responsible for one of these studies he mentioned—they had investigated a receptor important for the production of thyroid hormones, the so-called thyroid-stimulating hormone (TSH) receptor.
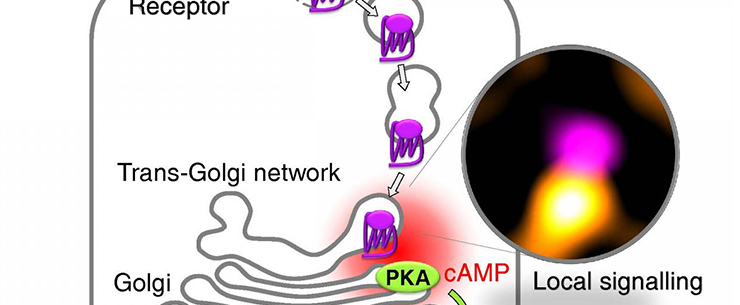
In the current study, the researchers at the University of Würzburg, together with colleagues from the University of Birmingham, succeeded in deciphering what is happening in the cell interior. As a major player, they identified the trans-Golgi network (TGN), a network of tubules and vesicles associated with the Golgi complex. This is the cell compartment where newly synthesized proteins are sorted into different transport vesicles that carry them to their final subcellular locations.
“Our new data show that the TGN is an important intracellular platform for the activity of G protein-coupled receptors,” noted Dr. Calebiro.
According to the scientists, their study reveals a new mechanism that explains the effects of GPCR signaling in the cell interior.
“We showed that upon internalization in thyroid cells, endogenous TSH receptors traffic retrogradely to the trans-Golgi network (TGN) and activate endogenous Gs-proteins in the retromer-coated compartment that brings them to the TGN,” the authors wrote. “Receptor internalization is associated with a late cAMP/protein kinase A (PKA) response at the Golgi/TGN. Blocking receptor internalization, inhibiting PKA II/interfering with its Golgi/TGN localization, silencing retromer or disrupting Golgi/TGN organization all impair efficient TSH-dependent cAMP response element binding protein (CREB) phosphorylation.”
The newly described mechanism of GPCR signaling inside thyroid cells can be recapitulated as follows: upon TSH binding, TSH receptors are taken up by the cells (internalized) and transported to the trans-Golgi network. There, the receptors induce the production of cAMP and activate another enzyme—protein kinase A. Since these events happen near the nucleus, where the genetic information of the cell is stored, they modify gene transcription.
“This study represents a significant step forward,” Dr. Calebiro concluded, as it presents a new model capable of explaining how GPCRs function inside our cells. These new results could “lead to the development of innovative drugs for a variety of human diseases that specifically target the uptake of receptors or their function in the TGN.”