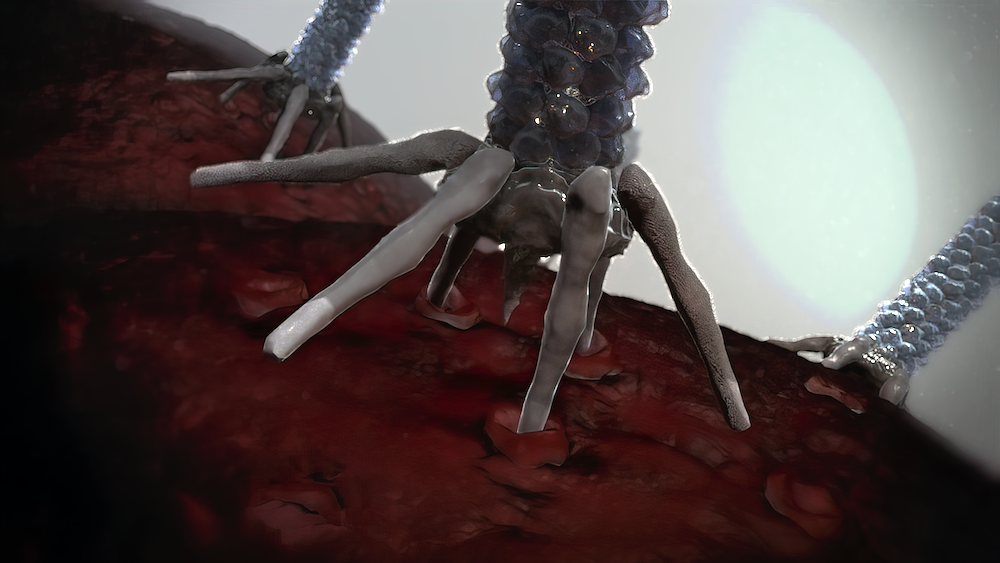
When Joseph “Joe” Healey saw a paper on “bacterial syringes” published in Nature last year, the list of co-authors led by his namesake, Joseph “Joe” Kreitz, made him think he was seeing double.
“When the paper came out, the most immediate reaction was just surprise,” Healey, the CEO of NanoSyrinx, told GEN Edge. “We know most of the groups working on similar things and related systems, so it was more surprising that it was on their radar and that they’d gotten no track record of working on this system. Our guess was that it was a student-driven project or something that they latched on to as they are such captivating systems.”

Healey’s suspicions were correct—Kreitz was a graduate student in the Broad Institute lab of Feng Zhang, PhD, who had dug deep into the literature upon learning about these peculiar virus-like syringes with protein delivery capabilities.
But after the initial shock and cycling of emotions, wondering if he’d just been scooped and if his IP was safe, Healey came to a feeling of success. “It was apparent very quickly that it’s a great paper—it’s amazing data,” said Healey. “Once the dust had settled, it became apparent that [the article is] helpful for us, because it is external validation. We are not just some cowboys in the lab that are working on this.”
Their initial funding sources and partners agreed that seeing robust data from another source was a huge support. Roughly ten months after the Zhang lab’s Nature paper, Healey arrived at his first J.P. Morgan Health Care conference (JPM) in San Francisco with his company’s chief business officer, James Lapworth, to seek additional backing for NanoSyrinx, the company based in Coventry, England, that he founded several years ago to turn “nanosyringes” into medicines.
Nearing the end of their seed round raised in 2021, the NanoSyrinx leadership is looking toward a series A round to build out and scale the nanosyringe manufacturing pipeline while simultaneously taking steps towards in vivo safety and efficacy studies.
“These [nanosyringes] have never been dosed before,” said Healey. “We’ve done some limited toxicity work, and we’ve got some efficacy studies going on. Ultimately, we want to take this as broad as we can to do multi-dose systemic delivery if possible. But there are all the usual questions about immunogenicity and distribution—these sorts of things that we just don’t completely know yet. The series A is certainly intended to at least round out that and give us a fuller picture.”
You never forget your PhD
Part of the reason that Healey took Kreitz’s data as exciting and not threatening is that he believes that he was the one who developed the payload manipulation aspect of the Photorhabdus bacteria with the contractile injection systems, research conducted during his PhD in the lab of Nicholas R. Waterfield, PhD, at the University of Warwick.
“That’s where the core IP for the company resides,” said Healey. “That was what came out of my PhD, specifically. We’ve been able to do that work for a couple of years now and then more recently with the [Broad] paper around retargeting. We were probably about six months away from [retargeting] in a slightly different way. That lays the groundwork for the platform itself.”

Since then, the NanoSyrinx team has been trying to get a handle on manufacturing these “weird, different, and unusual” bacterial injectors,” as Healey puts it. Besides their use of microbial fermentation, there’s no established solution for all aspects of manufacturing nanosyringes, let alone at CMC-grade levels for medical use.
Healey and colleagues have used synthetic and molecular biology to manipulate the payload platform to improve delivery yields and reduce toxicity. They’ve developed bioassays to show payload delivery and, in the past few months, have been gearing up to move beyond the proof of concept and into therapeutic modalities for their own pipeline and partnerships.

“We want to build our own value and assets, and we know that our venture investors want us to do that,” said Lapworth. “We also recognize that there’s a huge amount of good understanding out there of disease biology in some areas—we can’t do everything. We want to partner pretty early with the pharma people who have delivery challenges. They know what targets they want to hit; they know which cells [the targets] are inside, but they just don’t have a good way to do it yet.”
They’ve already started on that path, having recently announced a project with AstraZeneca that Healey and Lapworth say they cannot say much about.
“[AstraZeneca] nominated some payloads, and we went away, built them, and then tested them—and that worked,” said Lapworth. “We’re now talking about expanding that, but we’re also talking to other companies this week about starting to do more serious commercial projects. It’s really interesting to see people’s eyes light up when they recognize what this could potentially do. You can see the ideas sparking off in their heads.”
A looming IP wall?
The academic theory behind the nanosyringes of Photorhabdus bacteria is that they were phages once upon a time, millions of years ago, that eventually integrated into the bacterial genomes, where they’ve undergone selection pressures to develop different functions.
“One of the things that’s become more clear in the last 5–10 years is, originally, when Nick [Waterfield] first discovered these, for a long time, people just thought they were defunct phages—just junk,” said Healey. “There was no capsid, no genomic packaging apparatus. But now… we have a database of all of these types of [nanosyringe] structures, and they’re found across the tree of life doing all sorts of weird ecological tinkering and influencing.”
The unique selling proposition for nanosyringes is that the phage tail lookalikes should, theoretically, be easier, cheaper, and safer than viruses while delivering complicated payloads like proteins, enzymes, and toxins rather than genes.
“Even though this is bacterial in origin, we expect it’ll be less immunogenic than a co-opted human pathogenic virus,” said Healey. “Often, we have to tell people it’s like a phage because that’s the only thing they can immediately latch onto. It gives them a picture in their head straight to start to build from, but we’re always very clear that we haven’t just taken a phage and engineered it to deliver proteins. This is what evolution created for this purpose.”
What Healey finds most exciting about nanosyringes is the intracellular delivery of biological payloads like enzymes, proteins, and toxins because of their targetability.

“We realized potentially from the [Kreitz] paper and the work that we were doing that, actually, with the retargeting, there were more ways to skin that cat than there are with the payload loading,” said Healey. “If you had to flip a coin and pick one of those things to own, we think the payload stuff is the better thing to own. Even with the work from [the Broad], [targetability] is still a pretty nascent aspect of the technology—although we’d always hypothesized that it would be possible in the early days when we were starting the company because people have done similar things with phages and other systems in the past.”
It’s too early to see what will come of NanoSyrinx’s efforts to industrialize bacterial injectors for medical applications, let alone whether they’ll end up in patent battles with the Broad at some point. For now, these “wacky” inventions from nature are, according to Healey, “very, very early stage,” especially for the potential investors and partners they’ve been meeting at JPM.
Healey said that this was the case with their initial investors, but in the end, they got comfortable and bought in.
“[The investors] have been really good at helping us to focus in the very formative early days,” said Healey. “They’ve made sure that we focus on the right things and build the kind of data set that other pharma VCs and other pharma companies’ potential partners are going to care about. So having them and the other investors we have behind us and all able to follow on and underpin the next round helps people get comfortable that they’re not taking all the risks by themselves when they come into [nanosyringes].”
With so much still to learn about the platform, NanoSyrinx is still some distance away from any clinical utility of the bacterial injectors.
It’s unclear whether there’s been any movement on the Broad Institute front. The closest we’ve seen from Zhang’s side is the proprietary protein nanoparticles (PNPs) at his startup, Aera Therapeutics, which emerged in February 2023 with $193 million. But last week, Aera announced that it had laid off about 25% of its staff. Time will tell if Aera, another Zhang organization, or a Kreitz initiative will work on nanosyringes.
For now, NanoSyrinx is in the driver’s seat, riding its IP until it runs out of financial gas or a patent wall and crashes. For now, the plan is to go the distance.