
Technical Director
Charles River Laboratories
In recent years, we have seen great advances in disease diagnosis through biomarkers and, increasingly, gene sequencing. These advances enhance our ability to characterize diseases at the molecular level, and they allow us to uncover ever more subtle distinctions between cancerous cells and “normal” cells. Additionally, we have witnessed significant progress in data handling and artificial intelligence technologies. These technologies help us deepen our understanding of disease, and they support the development of novel therapeutic approaches and tools.
This progress has resulted in the development of a number of successful immuno-oncology approaches to cancer therapy such as checkpoint inhibitors and chimeric antigen receptor (CAR) T cell–based products. We are also seeing the development of cancer vaccines, which aim to train the immune system to target tumor-specific antigens.1–5 There are two approaches to these vaccines:
1. Targeting generic antigens found across patient subpopulations.
2. Targeting specific epitopes on the patient tumors.
The first approach leads to so-called off-the-shelf vaccines; the second approach, personalized vaccines.1 Although off-the-shelf vaccines are easier to manufacture, they require the incorporation of many irrelevant epitopes, potentially compromising efficacy. Also, they may benefit only selected patients. In contrast, personalized vaccines are potentially applicable across tumor types and for all patient populations. However, they can be difficult and costly to develop and manufacture.
The personalized approach is built around increasing sequencing capabilities and the use of algorithms to predict multiple MHC class I epitopes specific to an individual patient’s tumor. Then it is necessary to manufacture vaccines that are, essentially, targeted to an individual patient’s tumor. Doing so requires an extra degree of precision.
Despite these challenges, several companies, such as Roche/Genentech, Evaxion Biotech, EpiVax, and Geneos Therapeutics, have products that are in clinical trials or have already completed clinical trials. Product developers are now assessing a range of vaccine delivery approaches including peptides, plasmid DNA, and RNA. The selection will depend on both clinical aspects such as the number of epitopes the vaccines can target, as well as the ability to manufacture the vaccines.
The production of individualized medicines presents significant challenges to manufacturers but also creates scope for innovation in terms of manufacturing processes, equipment, facilities, and required quality systems.
A manufacturing perspective
The production of a personalized cancer vaccine proceeds as follows: biopsy the patient tumor; sequence the tumor DNA; apply artificial intelligence to identify antigen sequences; and manufacture, test, and release the vaccine. Cancer vaccines that have been produced to date include mutation-specific peptides, mRNA vaccines, and plasmid DNA vaccines (Figure 1).
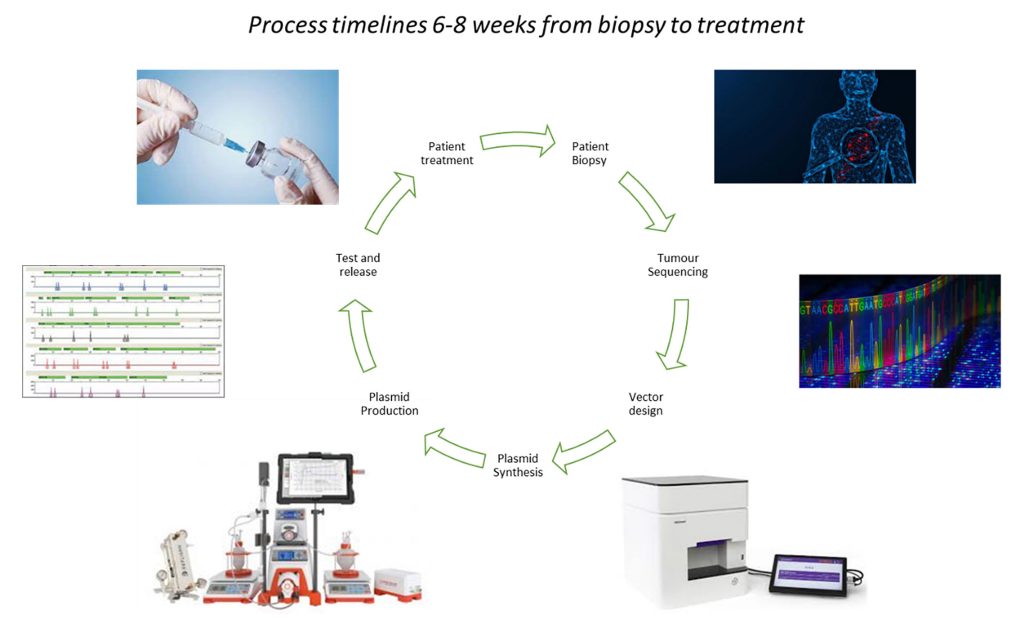
Personalized, or individualized, approaches are not unique to cancer vaccines. For example, these approaches are also being used for other products such as autologous cell therapies. However, personalized cancer vaccines pose unique manufacturing challenges.
One of these challenges is the need to address potentially large patient numbers. Cell therapy products such as CAR T-cell products have been targeted at small patient populations with resistant blood cancers, whereas cancer vaccines are currently targeting a broad range of patient groups, including patients with solid tumors. Manufacturing approaches must be able to sustain potentially thousands of “batches” per year, rather than tens or hundreds, which has significant implications for manufacturing.
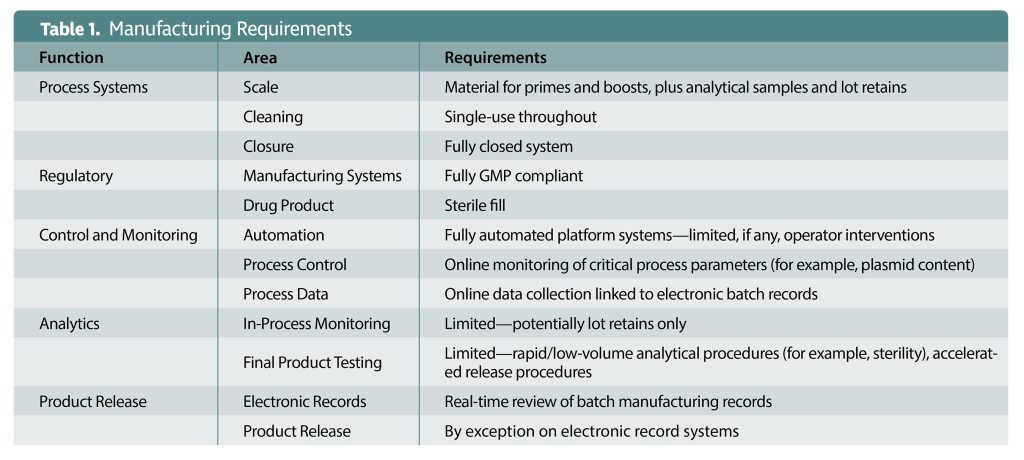
Another one of these challenges is the need to expedite the design, manufacture, and administration of the vaccines. The more time passes, the greater the clinical progression of a patient’s disease. Tumors will evolve to avoid the immune system, and the antigens on display will change. Consequently, vaccines must be designed, produced, tested, and released in an extremely short timeframe—weeks, not months. There is no scope for batch failures from a patient’s perspective. The whole manufacturing, testing, and release procedure will need to be reimagined to achieve the timelines and throughputs that will be required (Table 1).
 The two challenges cited thus far are important, but neither of them is as important as ensuring product safety. Control strategies must be put in place to segregate and trace products and to prevent cross-contamination. Additionally, process robustness is a prerequisite. Each product will be “unique” in terms of gene or peptide sequence, and there will be no scope for development studies to be performed. Consequently, the production platform must be validated and show that required yields and purity can be achieved with a wide range of model vaccine candidates. The process will also need to be streamlined to reduce production timelines. For example, it may be necessary to generate cell banks that can produce plasmid vaccines and other drug substances. Doing so can eliminate drug substance hold points.
The two challenges cited thus far are important, but neither of them is as important as ensuring product safety. Control strategies must be put in place to segregate and trace products and to prevent cross-contamination. Additionally, process robustness is a prerequisite. Each product will be “unique” in terms of gene or peptide sequence, and there will be no scope for development studies to be performed. Consequently, the production platform must be validated and show that required yields and purity can be achieved with a wide range of model vaccine candidates. The process will also need to be streamlined to reduce production timelines. For example, it may be necessary to generate cell banks that can produce plasmid vaccines and other drug substances. Doing so can eliminate drug substance hold points.
The scale of the equipment required for the manufacture of personalized vaccines is comparable to that required for laboratory systems. However, the equipment for personalized vaccines needs to be GMP compliant with respect to construction materials, traceability, and compatibility with single-use systems. Manufacturing for individualized therapies is also likely to create the need for integrated modular systems, rather than standalone process stages, with built-in sensors and monitoring to allow for fully automated control platforms, on-line data collection, and equipment operation. A highly standardized and robust material supply chain is also crucial.
From an operational perspective, eliminating the risk of contamination is of the utmost importance. Accordingly, facilities should be designed to enable:
- Segregation of batches.
- Integration of fully closed production systems.
- High-throughput production and rapid product changeover.
Innovations in the analysis of in-process and final products allow product testing to proceed more quickly and with smaller material volumes. However, with personalized vaccines, product testing must contend with the fact that each product is unique. Each product, then, may pose unique assay challenges. These may arise, for example, with identity, potency, and sterility assays.
Additionally, manufacturers will need high-throughput assays to test and release materials more quickly and to process samples in larger numbers. Manufacturers will also need to cope with all the retention samples that will be collected to test the profusion of personalized vaccines.
Regulatory considerations
The licensing and regulation of the production and release of personalized products is challenging and will require close relationships between drug developers, manufacturers, and regulatory authorities. This is not least to ensure that the product platforms are safe and that the required clinical thresholds are achieved. It will also ensure that the proposed manufacturing approaches are robust and able to consistently produce products of the right quality, independent of the vaccine “sequence.” Quality attributes will include product formulation and stability.
We must also consider regulatory, licensing, and quality management systems. They need to cover all stages of the manufacturing process from receipt of the initial plasmid through to the dispatch of material to clinic, including the supply chains for equipment and materials used in the production processes. There is also the issue of product release, which encompasses batch manufacturing record review and analytical testing. Product release needs to be streamlined, not least through the adoption of paperless approaches.
Final thoughts
In summary, we are entering a new era in cancer therapy with the concept of truly personalized, or indeed individualized, medicines. We now have the opportunity to exploit the huge advances in diagnostics and our knowledge base to develop truly individualized cancer therapies. However, if we are to realize this opportunity, there must be a revolution in manufacturing approaches.
References
1. Shetty K, Ott PA. Personal Neoantigen Vaccines for the Treatment of Cancer. Annu. Rev. Cancer Biol. 2021; 5: 259–276.
2. Scheetz L, Park KS, Li Q, et al. Engineering Patient-Specific Cancer Immunotherapies. Nat. Biomed. Eng. 2019; 3: 768–782.
3. Lo AA, Wallace A, Oreper D, et al. Indication-Specific Tumor Evolution and Its Impact on Neoantigen Targeting and Biomarkers for Individualized Cancer Immunotherapies. J. Immunother. Cancer 2021; 9(10): e003001.
4. Ingels J, De Cock L, Mayer RL, et al. Small-Scale Manufacturing of Neoantigen-Encoding Messenger RNA for Early-Phase Clinical Trials. Cytotherapy 2022; 24: 213–222.
5. Hu Z, Ott PA, Wu CJ. Towards Personalized, Tumour-Specific Therapeutic Vaccines for Cancer. Nat. Rev. Immunol. 2018; 18: 168–182.
Tony Hitchcock is the technical director for the cell and gene therapy development and manufacturing division at Charles River Laboratories.

