By Cenk Sumen, PhD

CSO, MaxCyte
From discovery to commercialization, the life cycle of biopharmaceuticals is driven by safety, affordability, and timeliness to market. Process optimization holds the key to shortening the development cycle and reducing cost of goods, all while adhering to the highest industry safety standards.
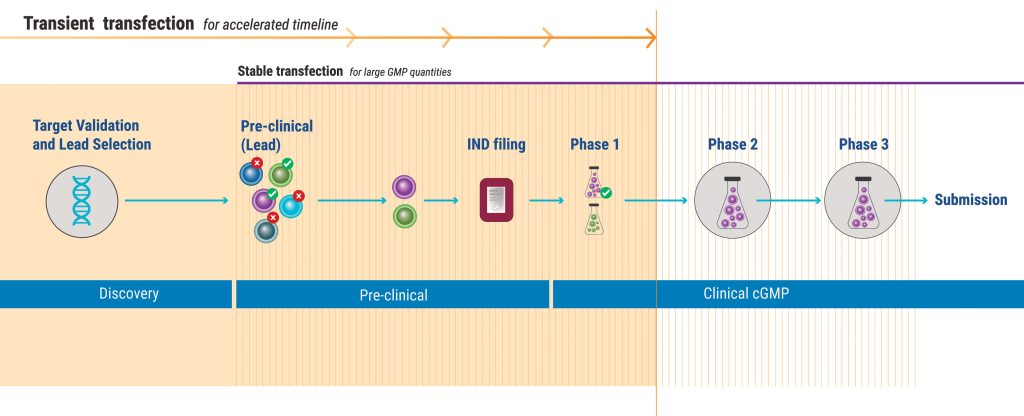
During biopharmaceutical development, cell lines that express specific proteins must be generated, typically by transfection. Employing transient protein expression—rather than stable protein expression—during more steps of the development process will enhance efficiency.
How things currently stand
Typically, therapeutic developers use transient protein expression only during the discovery phase. They invest early in stable cell line development before evaluating drug performance and ability to manufacture at scale.
During testing and validation, researchers typically evaluate a lead candidate for efficacy, toxicity, and manufacturability. If the protein fails, companies must invest more time, effort, and money to create an entirely different new stable cell line because the initial cell line cannot be repurposed for a different molecule.
Although stable cell lines have the virtue of enabling reliable, long-lasting protein production, establishing them takes significant time and effort, generating tremendous drag on innovation and constraining pipeline expansion. A lab might go through dozens of iterations before finding the right protein to take forward into the clinic. In the meantime, patients are waiting, competitors are moving forward, and the company is burning cash.
One relatively underutilized yet straightforward strategy is to employ transient transfection deeper into the development timeline. Transiently expressing cells can be significantly less expensive to develop than their stable counterparts. Even more importantly, getting a transient cell line to produce sufficient material for testing is a significantly faster process, taking weeks rather than months. This helps sponsor companies rapidly assess new molecules and choose which ones to take forward into the clinic.
Right now, it takes roughly 10 years or more, and costs around $1 billion, to develop one monoclonal antibody. Failure rates can exceed 90%. The biotech industry urgently needs to optimize therapeutic protein discovery, and adopting more effective cell lines can be a critical step in that process.
Faster, faster, faster
That life sciences research moves slowly is a given, but when world events brought a new sense of urgency, COVID-19 vaccines were developed with record-breaking speed. A process that had previously taken 10 years was completed in less than 2. This proves that research and development can respond with agility to a pressing patient need.
This new focus on speed is particularly important for companies working in novel spaces where being first to market offers unique advantages. Similarly, patients with limited therapeutic options may finally get a chance at treatment. Smaller patient populations and rare diseases can stand to benefit from this approach.
That’s why transient transfection is such a game-changer—it prioritizes speed and consistency. The technology utilizes electroporation to gently “relax” the cell membrane, delivering the genetic payload while enabling quick cell recovery. Newly engineered cells are ready to use in just a couple of weeks, and labs can produce sufficient amounts of many candidate proteins in parallel for testing.
In other words, this approach gives researchers new opportunities to “fail” quickly—that is, to abandon fruitless options quickly—and turn to potentially successful options. This is a critical paradigm shift. Failing quickly is difficult when depending on stable cell lines because companies must make sizable long-term investments just to produce them.
Transient transfection gives labs the ability to rapidly iterate. When a molecule doesn’t work, labs can move quickly to the next molecule and, if necessary, the one after that. Labs are no longer bogged down for months pursuing stable transfection.
This accelerated timeline means that companies can expedite decision making and dramatically reduce costs. Once the new candidate is proven safe and effective, labs can stably transfect a cell line to complete optimization and move into commercial production.
Implementing transient transfection
Established practices can be hard to change. People and organizations are sometimes hesitant to consider new options, even when the existing process has flaws.
Fortunately, transient transfection has already been widely adopted, on a small scale, in the early stages of the biopharmaceutical development process. The consistency and reproducibility of electroporation can smooth the transition to larger-scale production.
The regulatory pathway is also an important concern. Many people believe that stably transfected cell lines provide the best, perhaps the only, path to regulatory approval. However, there is precedent in vaccines, many of which use transient transfection all the way through to commercial production.
In addition, electroporation is already being used in FDA-approved cell therapy trials, which are, in many ways, more complex than bioproduction. There are also commercially available therapeutics being made in transient cell lines. The technology has been proven effective and has a viable regulatory path.
To the victors go the spoils
While virtually any company engaged in bioproduction can benefit from transient transfection, this technology could be particularly useful for biotech startups and contract development and manufacturing organizations (CDMOs).
CDMOs must constantly scan the horizon for competitive advantages. Accelerating production and reducing costs give them a competitive edge. For startups, the problem of improving time to market is ever present, and transient transfection can be part of the solution.
If we want to develop a faster, more affordable, and effective therapeutic protein pipeline, we are going to have to take some rational risks. Leveraging the unmet potential of transient transfection is a perfect starting point. It is proven technology that is poised to scale up, and in the end, the benefits to patients could be transformative.
Cenk Sumen, PhD, is the chief scientific officer of MaxCyte.




