Single-use technologies gather lots of today’s bioprocessing headlines. People in the field know the benefits, but there are potential drawbacks. As Noemí Dorival–García, PhD, a research fellow at the characterization and comparability laboratory at the National Institute for Bioprocessing Research and Training in Dublin and her colleagues wrote: “Despite their advantages, these plastic assemblies draw concern because they are a potential source of contamination due to extractable and leachable compounds.” These scientists developed a sensitive method—a combination of accelerated solvent extraction (ASE), ultra-high-performance liquid chromatography (uHPLC), and mass spectrometry (MS)—for identifying and quantifying compounds in the plastic materials employed in single-use bioprocessing.
To test these plastics, the Product Quality Research Institute (PQRI) recommends that controlled extraction studies should include multiple solvents of varying polarity. “These recommendations require many extractions and associated characterization, as no single extraction technique and method can solubilize all potentially relevant extractables,” Dorival–García explains. “In this context, ASE is an excellent alternative to other extraction techniques, as ASE does not have limitations in the type of solvent that can be applied, as occurs with other techniques such as Soxhlet or microwave extraction.” In addition, ASE requires less organic solvent and time than other methods. As Dorival–García points out, this reduces solvent “requirements from hundreds to tens of milliliters, and ASE requires less extraction time, minutes rather than hours.”
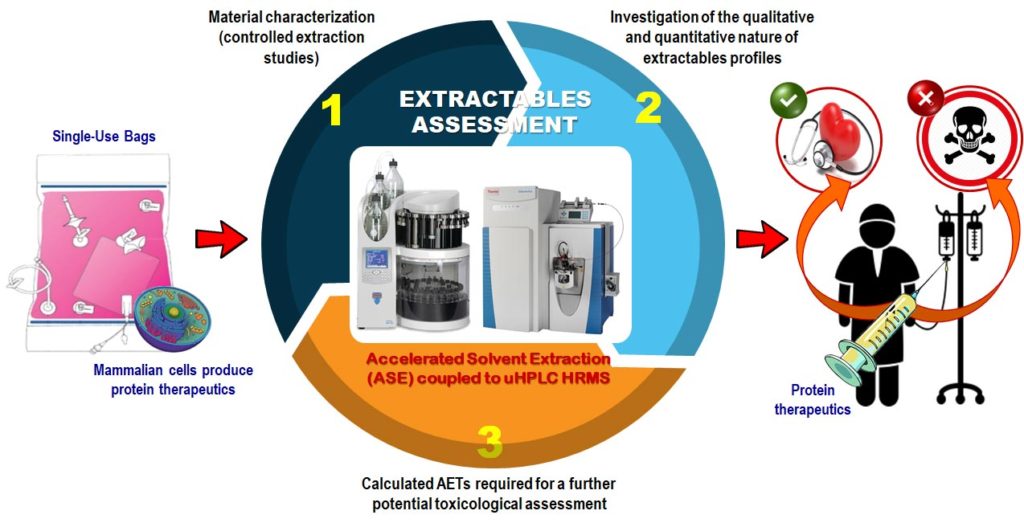
Dorival–García and her colleagues developed what she calls “a comprehensive workflow for the optimization of ASE-extraction parameters for single-use bioreactor bags.” She adds that they also described “a non-targeted analytical strategy that is focused on the identification of extractables, and how to interpret the results produced by extraction with different solvents.”
Still, Dorival–García sees more to explore about the additives in single-use bioprocessing bags. “It will be important to generate knowledge about toxic effects that the different classes of additives can produce on mammalian cells used to produce therapeutic proteins,” she says. “Such effects are only partially known, especially when considering the degradation or transformation product from extractable and leachable compounds in the bags.”
This is all just the start of what Dorival–García is planning, and the safety of future biotherapeutics could depend on the results. As Dorival–García says, “Our research group is aware of the significant responsibility of developing analytical methods that balance excellent performance with practicality—that is, simple, cheap, automated, etcetera—because the answers to all these questions about the role and impact of additives from single-use components on cells or humans and the pharmaceutical industry rely on our ability of identify and quantify these extractables and leachables.”