Intensified processing has become significantly more prevalent in the biopharmaceutical industry over the past few years. By lowering production cost of goods and increasing flexibility in plant design and utilization, newer processes yield tremendous improvements in efficiency, quality, and output, offering the promise of improved global access of biotherapeutics for patients.
For upstream process intensification, Chinese hamster ovary (CHO) cells continue to be the cell line of choice for production of protein-based biotherapeutics. Attention has now focused on identifying CHO cell lines that are capable of high-cell-density cultivation over increasingly longer culture times, while also producing consistent product quality and titer. However, CHO clones, which perform well in fed-batch culture, cannot be assumed to also perform well under intensified process conditions; protein expression levels and genetic stability may vary considerably when cultured at high cell densities for longer process runs.
Therefore, during the initial stages of intensified processing, it is essential to identify the most productive and/or stable clones prior to scale-up studies. This requires screening large numbers of clones, which can be time consuming and resource intensive if performed in shake flasks and benchtop bioreactors. The use of small-scale microbioreactor systems, capable of running as high-inoculation fed-batch and/or perfusion mimics, provides a way to implement a high-throughput solution to accelerate clone/media selection for upstream intensified process development.
Performance comparison of intensified approaches
The ambr® 15 cell culture, an automated microbioreactor system launched by Sartorius Stedim Biotech in 2010, has established itself as an effective, small-scale screening system for clone selection and early process optimization. The system comprises an automated workstation, a laptop computer with control software, and single-use cell culture microbioreactors with 10–15-mL working volumes. Installed in a biological safety cabinet for aseptic operation, ambr® 15 monitors and controls 24 or 48 microbioreactor cultures in parallel.
Studies in ambr® 15 cell culture have shown improved results when compared to shake flask or shaking plate cultures due to the high level of automation combined with reliable and independent process control for pH and dissolved oxygen. Furthermore, the system provides predictive performance for benchtop bioreactors. With low working volumes, the small-scale stirred bioreactor vessels offer considerable advantages for screening cell lines and media when running intensified processes, particularly in terms of costs.
Recently, the system has been used as a perfusion mimic for intensified processing by Sartorius Stedim Biotech’s Cell Line Development team at Ulm, Germany. Data presented at the European Society of Animal Cell Technology Conference in 2019 compared the same CHO cell clones cultured in standard fed-batch bioreactors (5 L), high-inoculation fed-batch bioreactors (1 L), and ambr® 15 microbioreactors (10–15 mL) under perfusion mimic conditions using 1 vessel volume/day (VVD) media exchange. The results showed that cells cultured in standard fed-batch conditions achieved a mean titer of 0.35 g/(L/d) compared to 0.94 g/(L/d) in high-inoculation fed-batch conditions, 2.7 times higher than standard culture. Perfusion culture showed the best results, however, producing 1.41 g/(L/d), over 4 times higher than standard fed-batch culture.
To investigate the performance of various CHO clones expressing different products under perfusion mimic conditions, seven CHO clones were cultured in parallel and compared to titers achieved previously when run in fed-batch processes. The clones were designated CL1–CL7 and were grown in spin tubes (max. 35 mL working volume) under perfusion mimic conditions using 1 VVD media exchange for 14 days.
The results (Figure 1) show that volumetric productivity increased for most clones when compared to their fed-batch reference counterparts; however, the response to process intensification varied across the different clones. Some clones exhibited only a marginal titer increase (CL5 and CL7), while other clones (CL2 and CL4) responded better, with up to threefold higher volumetric productivity. Only one clone (CL3) exhibited reduced productivity.
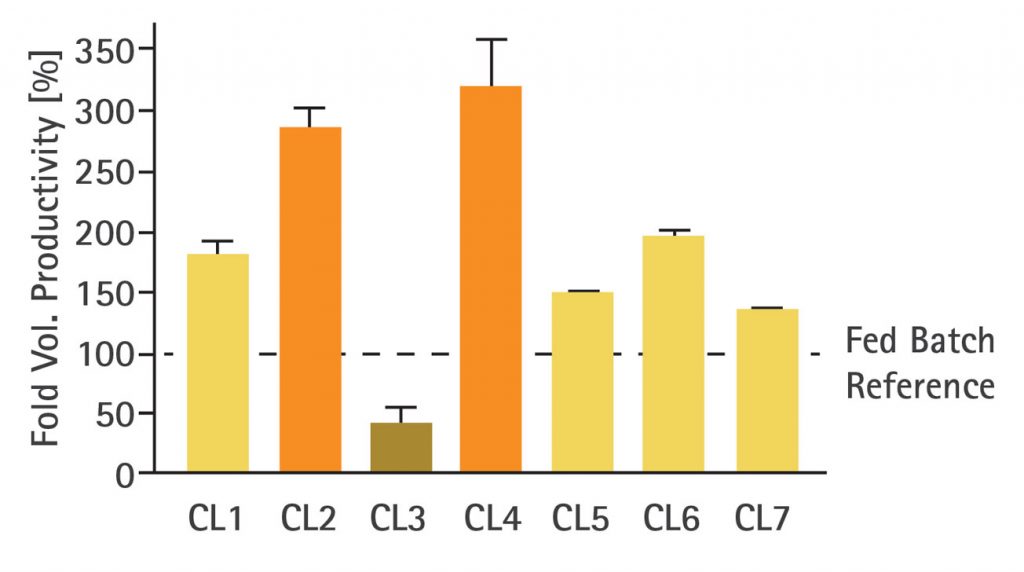
As demonstrated in this study, the response to process intensification was not the same across the different clones and did not always provide an improvement in volumetric productivity, meaning that some clones previously selected for their performance in fed-batch were not suitable for perfusion culture. These results indicate that dedicated screening is advisable to ensure the best-performing clones are selected under relevant process conditions.
Perfusion mimic
The ambr® 15 cell culture Generation 2 system, launched in 2019 as the successor to the original system, has undergone a redesign to improve performance, increase flexibility, and add new functionality to support intensified processes. One of the new features, the Rapid Vessel Drain, provides the capability to bleed large volumes of culture and quickly remove spent media from the ambr® 15’s microbioreactors. In addition, the latest ambr software includes novel process steps to automate perfusion mimic protocols.
Two perfusion mimic approaches have been established in the ambr® 15 cell culture system—a centrifugation method and a cell settling method. The microbioreactor vessels do not have any cell retention devices; therefore, cells are allowed to settle either by gravity or by centrifugal force. When the new Rapid Vessel Drain functionality is used, removal of spent media from microbioreactors following either centrifugation or cell settling steps is easily managed by the liquid handler. The Rapid Vessel Drain setup reduces the time to remove spent media from all the microbioreactors in a culture station by up to 90% (process time is reduced from around 2 hours to just 12 minutes).
For clone stability studies, the system can also be set up with fully automated serial passaging in the microbioreactors. New ambr® clone selection software allows simplified, streamlined multivariate data analysis for faster, more consistent cell line screening and ranking. The combination of these features and software provide a scaled-down bioreactor model which supports Design of Experiments for intensified processing.
Perfusion culture mimic with centrifugation
Centrifugation is the most common method of separating cells from supernatant, where vessels are centrifuged to create a cell pellet and the remaining supernatant is exchanged with fresh media at a 1 VVD. The steps in which vessels are out of process control were performed in rapid succession to minimize process disruption time.
With the ambr® 15 cell culture Generation 2 system, supernatant was removed using the Rapid Vessel Drain, which allowed a quick (~5 seconds) aspiration of the entire supernatant from each vessel. Cell bleeding was performed using the Passage Culture Vessel step, which removed a volume of culture from the vessel and replaced it with fresh media to reach a specified viable cell density (VCD = 20 × 106 cells/mL).
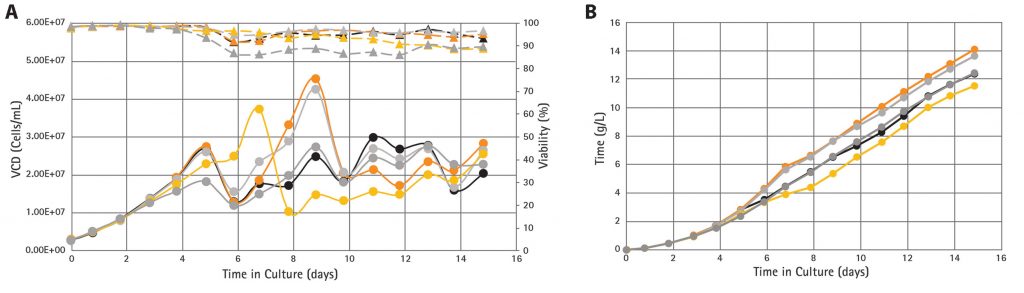
For centrifugation, ambr® 15 centrifuge adapters were used, each with the capacity to hold three microbioreactor vessels, allowing up to 12 to be centrifuged simultaneously. The angled design ensured that cells were pelleted in the corner of the vessel farthest away from the pH and dissolved oxygen sensors and the sampling port, allowing easy supernatant removal using the Rapid Vessel Drain function with minimal disturbance to the cell pellet. As cell pellets grew larger due to increasing cell numbers over the course of the experiment, the relative centrifugal force was also increased to retain cells in the vessel.
The results (Figure 2A) show some variability in growth profiles due to cell bleeding steps before day 10. However, after day 10, once the cell bleed process had stabilized, cells showed good overall reproducibility for growth (maintaining VCDs at approximately 20 × 106 cells/mL) and high cell viabilities (85–95%). The cumulative product titers reached 12–14 g/L (Figure 2B), and the average daily titer from day 5 onwards reached 1.0 g/L (with titer differences attributable to variability in growth).
Perfusion mimic with cell settling
Cell settling is another method of separating cells from the supernatant, accomplished by switching off stirring in the microbioreactors and allowing the cells to settle to the bottom of the bioreactor before exchanging a portion of the supernatant with fresh media. A settling time of 35 minutes and media exchange three times per day (one third of the working volume every 8 hours for a 1 VVD perfusion rate) was found to provide the best conditions to minimize cell loss and maintain good cell viability for the particular CHO cell line and process used in this study.
Sample removal for cell counts was performed once daily between settling events, as was cell bleeding. Cell bleeding was performed using the Passage Culture Vessel process step, which exchanges a certain volume of culture with fresh media to target a specified viable cell density (VCD = 20 × 106 cells/mL) using the Rapid Vessel Drain.
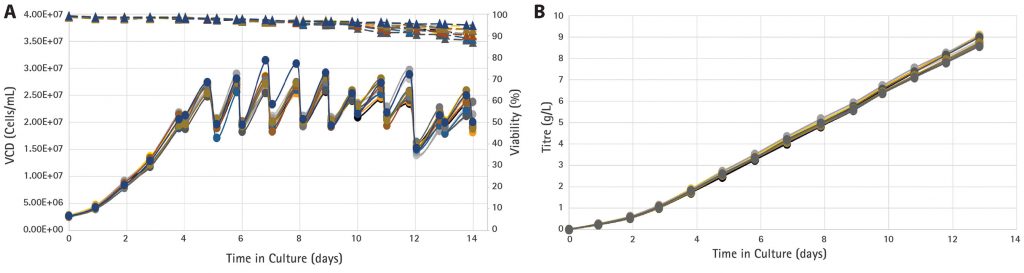
At each settling, titer samples were taken after 22 minutes from an appropriate height to remove cell-free samples. After 35 minutes, the remaining top third of the culture volume was removed from each microbioreactor using the Rapid Vessel Drain; stirring and other process controls were restarted; and fresh media was added back to each vessel to take the working volumes back to 10 mL. The total settling time, including media refill, was 55 minutes for an entire culture station comprising 12 microbioreactors. Steps for culture removal and media addition were written into ambr® 15 software and automated so that these steps could run overnight with no manual interactions or supervision.
The results (Figure 3A) show excellent reproducibility for cell growth and viability across all microbioreactors, maintaining VCDs at approximately 20 × 106 cells/mL and high cell viabilities (85–95%) throughout culture duration. The cumulative product titers were consistent across all bioreactors, reaching 8 to 9 g/L (Figure 3B) over 13 days, and the average daily titer from day 5 onwards reached 0.8 g/L.
Conclusions
CHO clones cultured in the ambr® 15 cell culture system under perfusion mimic conditions can show significant increases in titer of up to fourfold on standard fed-batch conditions, and productivity improvements of up to threefold using CHO clones from fed-batch selection. The system also enables the identification of clones with limited performance gains or risk of low productivity in intensified setups.
The ambr® 15 cell culture Generation 2 system offers improved performance and new features for running efficiently in perfusion mimic mode with two different approaches highlighted—centrifugation and cell settling. The system is capable of maintaining cultures at target VCDs with the use of the Rapid Vessel Drain, automated at-line cell counting, and automated cell bleed steps. The results showed a high level of consistency and reproducibility across the different experiments for VCD, cell viabilities, and product titers over a period of 15 days.
In summary, ambr® 15 cell culture can facilitate high-throughput perfusion mimic studies at the microscale with reproducible results to enable selection of suitable clones for process intensification. This could help to rapidly identify the best-performing clones at the early stages of process development, to cost-effectively support larger scale intensified processes, and, ultimately result in the manufacture of more affordable biotherapeutics.
Alison Rees-Manley ([email protected]) is ambr 15 product manager at at Sartorius Stedim Biotech.


