Sponsored content brought to you by
Cardiovascular diseases are the number one cause of death globally. They include conditions such as coronary heart disease (CHD), heart attack, high blood pressure, arrhythmias, congenital heart defects, vascular dementia, and stroke. In Europe alone, cardiovascular diseases cause over 4 million deaths each year.1 In humans and other vertebrates, the cardiovascular system is vital for maintaining overall health and well-being. This intricate circulatory network delivers oxygen, nutrients, and hormones to different parts of the body while also removing waste products. However, cardiovascular diseases can develop due to factors like genetics, lifestyle choices, and environmental effects.
Cardiovascular research helps to improve our knowledge of the cardiovascular system. In addition to revealing the underlying causative mechanisms, possible targets for the prevention and treatment of cardiovascular diseases can also be identified. The ultimate goal of cardiovascular research is to improve individual patient outcomes and reduce the burden of disease on society.
The most common underlying pathology of cardiovascular diseases is atherosclerosis. Atherosclerosis involves the narrowing of the arteries due to a gradual buildup of fatty material on the interior surface of the vessels. The narrowing of the arteries makes it harder for the blood to flow through the circulatory system, which reduces the oxygen supply to cells. Cardiac ischemia refers to when heart muscle cells are affected by this lack of oxygen. A reduced oxygen supply to cells results in mitochondrial dysfunction and oxidative stress and causes inflammation. If a fragment of fatty material breaks away from a plaque, it can cause a complete blockage of the vessel. This leads to the death of the surrounding cells. If such a blockage occurs in the heart, dying muscle cells may cause a heart attack (myocardial infarction). If a plaque fragment leads to a blockage in a tissue or organ other than the heart, it can lead to a stroke (brain ischemia); ischemia of the skin, small intestine, or kidney; or other diseases in different organs. An ischemic event can also be followed by vascular decongestion with the re-establishment of blood supply (reperfusion) in an infarcted organ or tissue. Paradoxically, the restoration of the blood supply causes additional tissue damage, mainly because of induced oxidative stress and inflammatory responses. This sequence of changing oxygen supply levels is called “ischemia-reperfusion.” Understanding the physiology of ischemia and reperfusion injury is a key area of clinical interest. So far, however, it has been difficult to initiate, maintain and reverse acute hypoxic environments in in vitro studies.
Studying ischemia-reperfusion events
Ischemia-reperfusion research has advanced our understanding of the underlying mechanisms of cardiovascular diseases and permitted testing of new strategies to reduce tissue damage. However, this type of research primarily relies on small animal models. In addition to ethical concerns, this type of research is expensive and limited in throughput. In vitro models represent an important alternative to in vivo models since they can highlight the direct effect of reperfusion itself and the impact of a drug on specific cells.
Microplate-based analysis enables a substantial increase in throughput. Different microplate readers from different manufacturers allow control of the environment inside the reader. The levels of CO2 and O2 can typically be controlled from 1 to 20%. In some cases, O2 can decrease to 0.1%. Nevertheless, most plate readers can cover only the ischemic phase but not the reperfusion part. The CLARIOstar® Plus from BMG LABTECH with Atmospheric Control Unit (ACU) is the only microplate reader on the market that can rapidly re-oxygenate the measurement chamber of the reader to simulate reperfusion by active venting.
As shown in Figure 1A, the CLARIOstar Plus with ACU facilitates precise atmospheric control (grey dots/line) from room O2 levels to 1% O2 in less than 10 min, a defined time at 1% O2, followed by a rapid reperfusion to 18% O2 (gas ramping). Here, the ischemia-reperfusion experimental setup was used to study the oxygenation level of cardiomyocytes with the time-resolved fluorescence O2 probe MitoXpress Intra (Agilent Technologies), which measures intracellular oxygenation in real time. The experiment used cardiomyocytes derived from induced pluripotent stem cells (iPSC), which are considered one of the most physiologically relevant models available for use in drug screening.2 Part of the cells were treated with antimycin (purple dots/line) which inhibits mitochondrial electron transport and thereby blocks cellular respiratory activity. Nonrespiring cells reflect ACU conditions, while untreated, respiring cells (green dots/line) experience significantly reduced O2 concentrations. Cellular respiration significantly impacts oxygen concentrations at the cell monolayer. Respiring cells experience much lower resting oxygen concentrations and a more sustained hypoxia. This important finding for cardiovascular research highlights the importance of real-time oxygenation monitoring, which is made possible by the ramping function and the various detection options of the CLARIOstar Plus.
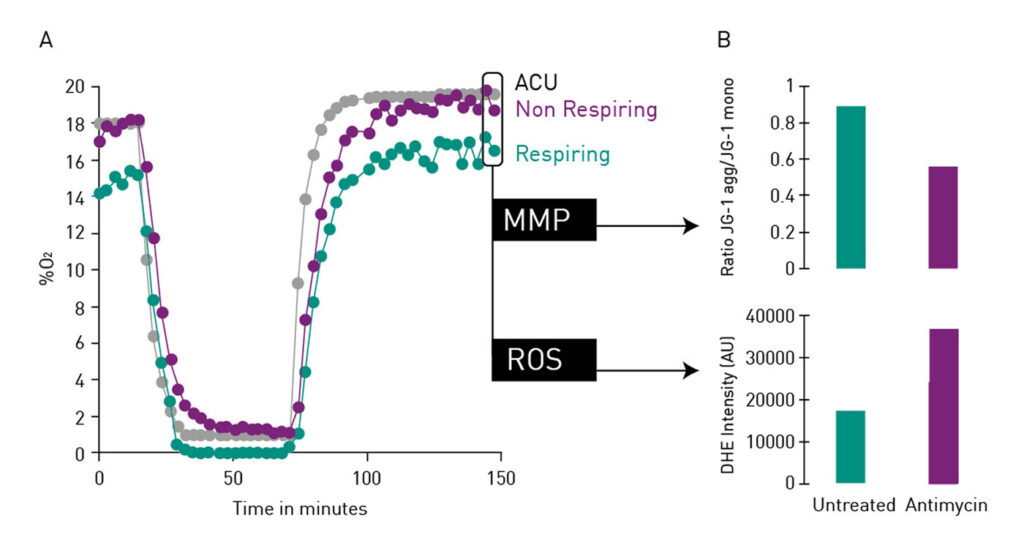
The convenient multiplexing function of the CLARIOstar Plus further facilitated analysis of the mitochondrial membrane potential (MMP) and the generation of reactive oxygen species (ROS) as a measurement of oxidative stress in parallel to cellular oxygenation levels. MMP was studied by detection of the JC-1 (Cayman Chemical) aggregate/monomer ratio, while generated ROS were detected with dihydroethidium (DHE; Sigma Aldrich). This allowed simultaneous detection of the increased dissipation of MMP and the elevated generation of ROS by respiratory cells (Figure 1B).
Predicting drug-related cardiac side effects
Cardiovascular research can be used to examine pathological processes and to develop targeted medications for therapy. It can also seek to predict the potential unwanted side effects of drugs on the cardiovascular system regardless of their actual target site. Here, in vitro assays that mimic the in vivo environment as accurately as possible are also critical to ensure reliable and predictive toxicology data.
Calcium (Ca2+) is a crucial regulator of cardiac myocyte function. Ca2+ links the electrical signals that pervade the heart with the contraction of myocytes. Cellular calcium channels may open when a signal like depolarization of the membrane potential takes place.
This leads to a release of calcium into the cytoplasm.
Calcium flux assays use various calcium indicators, which are often fluorescent or luminescent, to monitor calcium release. These indicators can be combined for use in real-time approaches in intact cells. Rhod-4 is an example of one of these fluorescent calcium sensors. The fluorescence of Rhod-4 increases substantially when associated with calcium. In the following approach, the dye was used to study the calcium flux and hence the beating rate of iPSC-derived cardiomyocytes upon treatment with isoproterenol or vehicle. As shown in Figure 2, the treatment of iPSC-derived cardiomyocytes with isoproterenol more than doubles the beating rate of cells. The binding of Rhod-4 to released calcium was monitored with the fluorescence detection mode of the CLARIOstar Plus. The reader enables ultra-fast data sampling rates of 100 measurements/second and is further equipped with the most sophisticated direct bottom optic for best detection in adherent cell samples.

Conclusion
Successful cardiovascular research depends heavily on the availability of convenient physiologically relevant models. The CLARIOstar Plus with ACU makes it possible to culture relevant cell models under in vivo conditions by mimicking not only hypoxic conditions but also reperfusion. The reader’s fast sampling rate and multiplexing ability further support the generation of reliable data to reveal the underlying mechanisms that cause cardiovascular diseases. These features also allow the discovery of new targets for the treatment and prevention of cardiovascular diseases.
References
- Townsend N, Wilson N, Bhatnagar P, et al. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016; 37(42): 3232–3245. DOI: 10.1093/eurheartj/ehw334.
- Ebert AD, Svendsen CN., Human stem cells and drug screening: opportunities and challenges. Nat Rev Drug Discov. 2010; 9(5): 367–372. DOI: 10.1038/nrd3000.

If you are interested in further details about microplate-based cardiovascular research, please contact us at [email protected]