Sponsored content brought to you by
Antigen-presenting dendritic cells (DCs) act as messengers between the innate and the adaptive immune systems. Present in tissues that are in contact with the external environment, such as the skin, the inner lining of the nose, lungs, stomach, and intestines, DCs can also be found in an immature state in the blood in very low concentrations (<1%). For in vitro studies, they are typically generated from monocytes.
DCs are indispensable in immunology and immunotherapy research on cancer and infectious diseases, as well as autoimmune disease and transplant rejection. As key elements of personalized vaccines for cancer and infectious diseases, they advantageously generate immunological memory and allow for autologous or allogeneic therapies, which have demonstrated promising results in the clinic.
Despite heavy investment in R&D involving DCs, under-developed and inefficient manual protocols for DC generation lead to batch-to-batch variability and inconsistencies and contribute to increased costs and research slowdowns.
The current gold-standard method for generating DCs from monocytes has 11 laborious manual steps involving 15 hours of technician time. Steps include static culture and stimulation with cytokines contained in culture media using multiple T-flasks or well plates.
To address this manufacturing dilemma, market-leading Corning entered into an agreement with Flaskworks to commercialize MicroDEN, an automated fluidic system that allows for differentiation of monocytes into DCs utilizing continuous perfusion of differentiation media.
“Using an automated, perfusion-based process such as MicroDEN for dendritic cell production eliminates six manual steps, reduces the risk of contamination, and efficiently produces repeatable results in less time,” said Elizabeth Misleh, senior product line manager, bioprocess, at Corning Life Sciences. “It also eliminates the variability that can result from different levels of technician proficiency.”
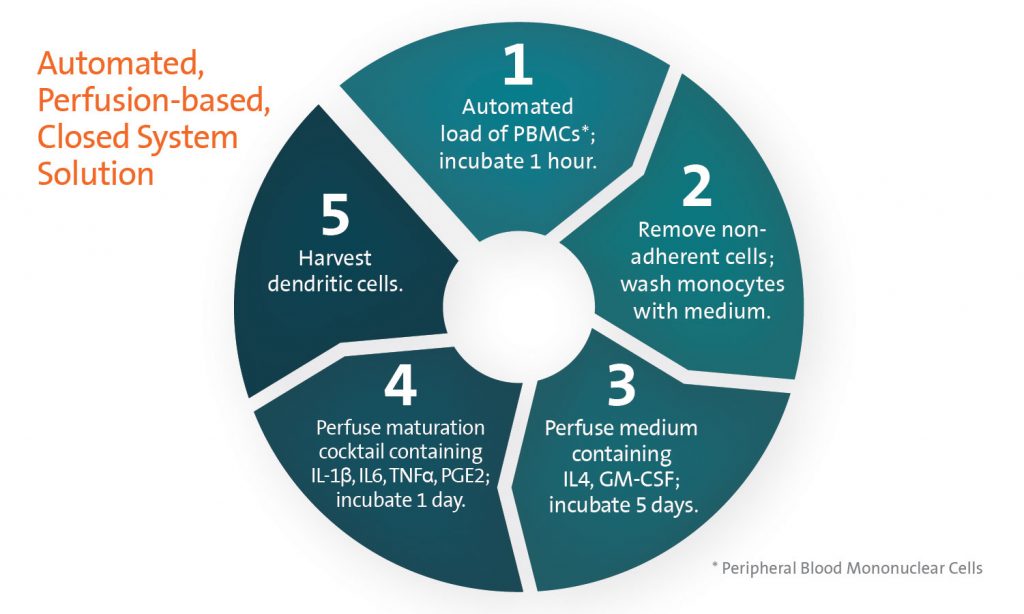
The closed system provides consistent yields and requires only three hours of technician time during the week-long process (see figure). MicroDEN leverages the benefits of a polystyrene culture surface, similar to the manual method, to provide for removal of non-adherent cells. Up to 25 million DCs can be reproducibly generated per unit, an equivalent or greater yield than manual production.
Benchmark phenotyping along with allogeneic T-cell proliferation and syngeneic antigen-specific functional assays demonstrated no phenotypic or functional differences based on manufacturing methodology.1 The same conventional phenotypes also resulted using culture medium containing either FBS or human serum, and when using serum-free medium.
In fact, in an allogeneic functional assay with immature DCs co-cultured with allogeneic CD3+ T cells for five days, MicroDEN-generated DCs proved better than manually generated cells at inducing T-cell proliferation. Proliferation was analyzed using CellTraceTM Far Red.
The RUO system will quickly demonstrate its superior approach to generation of DCs in routinely performed workflows and assays. Applications for MicroDEN-generated DCs include tumor-infiltrating lymphocyte (TIL) workflows that require a screening step, typically performed with autologous, manually produced, monocyte-derived DCs, for selection of appropriate subtypes and validation of neoantigen-specific T-cell receptors (TCRs).
To illustrate another example, a reliable supply of DCs is also needed for mixed lymphocyte reactions (MLRs) and antibody dependent cellular cytotoxicity (ADCC) assays that are widely used in screening of biologic and small molecule drugs. Higher T-cell proliferation and production of relevant cytokines are screens for antitumor potential.
It is no longer necessary to invest months to train technicians to manufacture DCs and still receive inconsistent results that vary batch to batch. The innovative MicroDEN perfusion system eliminates 55% of the manual steps, and delivers reproducible results in less time while greatly reducing contamination risk. Now, Corning and Flaskworks have shown that there is indeed a better way to manufacture DCs.
Reference
1. Automated generation of immature dendritic cells in a single-use system. Kozbial A. et al. J. Immunol. Methods 2018 June; 457: 53–65. doi:10.1016/j.jim.2018.03.010.
For more information and to request a MicroDEN demonstration, corning.com/MicroDEN