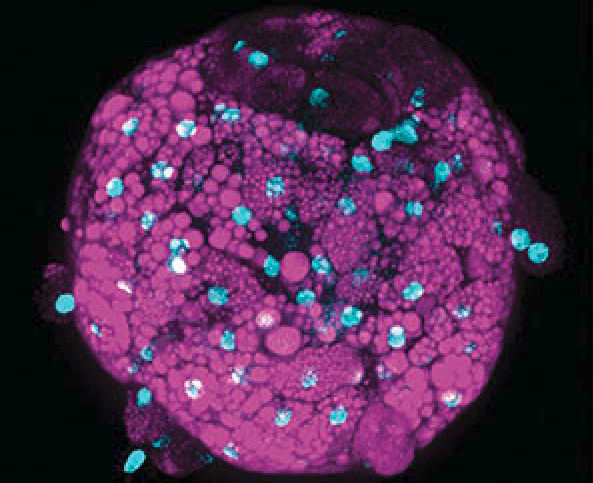
Preclinical model systems are invaluable tools for drug discovery and development. But many complex diseases, such as nonalcoholic steatohepatitis (NASH), are difficult to recapitulate in the laboratory. NASH is a progressive metabolic disease that takes years, if not decades, to develop in humans. Successfully modeling all the key physiological events (from steatosis to inflammation and fibrosis) evident in clinical NASH has been extremely challenging in both in vivo animal models and in vitro cell-based models.
Why? Conventional in vitro models, such as human hepatocytes cultured in 2D, offer a fast, efficient means to assess certain characteristics of NASH with compound testing (for example, the compound’s EC50), but they lack other cell types involved in the disease’s progression and are often suitable only for short-term assays. More advanced tissue-specific, human 3D co-culture systems, such as InSphero’s 3D InSight™ Human Liver Microtissues, are more appropriate for evaluating compound mechanisms of action in long-term experiments, but they, too, are limited in their ability to mimic NASH progression.
Diet-induced and genetically engineered mouse models are currently the gold standard for NASH research, but they are expensive, typically require 20 or more weeks to show the first hallmarks of NASH, and are unsuitable for high-throughput screening. In addition, mice have known species-specific differences that don’t translate to human biology.
More complex, disease-specific in vitro systems, such as InSphero’s 3D InSight™ Human Liver NASH Model, serve to bridge the gap between standard preclinical models and human clinical trials (Figure 1). InSphero’s NASH model is precisely engineered to incorporate all relevant human liver cell types (primary human hepatocytes, Kupffer cells, hepatic stellate cells, and liver endothelial cells) required for NASH induction and progression. It includes rigorously tested inducers for in vitro recapitulation of the three stages of NASH (steatosis, inflammation, and fibrosis) within 10 days. Although it can’t reproduce the systemic interactions of multiple organs involved in metabolic syndrome, it is appropriate for compound screening, deciphering disease-underlying mechanisms, and testing potential therapeutics prior to advancing them to clinical studies.
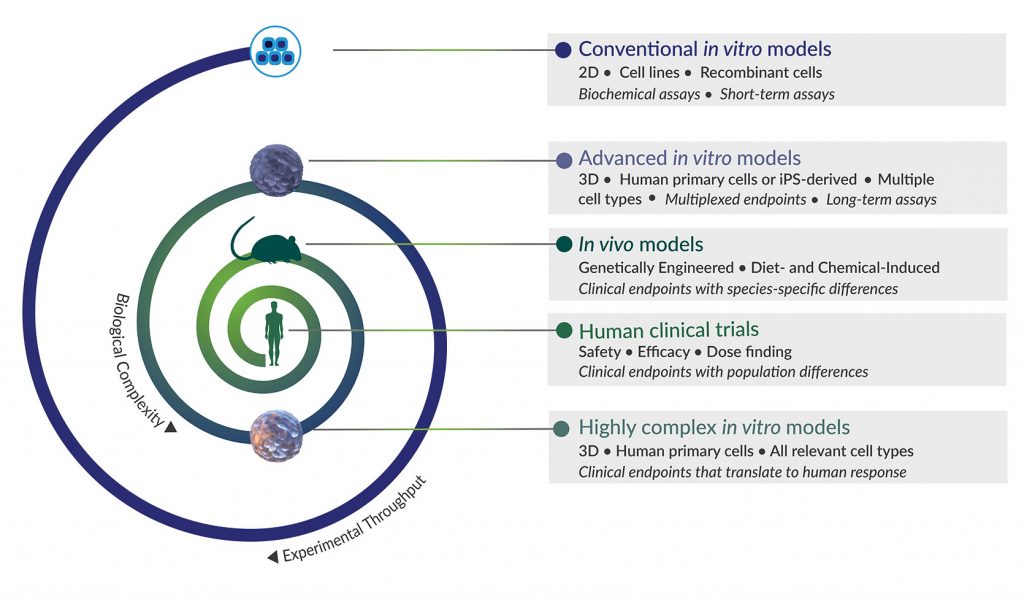
Collectively, these diverse models provide researchers a more comprehensive and predictive suite of tools for NASH drug discovery. In fact, several model systems may need to be employed for orthogonal or sequential validation of a drug candidate’s specificity, efficacy, and safety. How do you choose the most suitable model at each stage of drug development? By clearly identifying the type of information you need and the type of experiments to run in the context of the experimental question you want to answer at that stage (Figure 2).
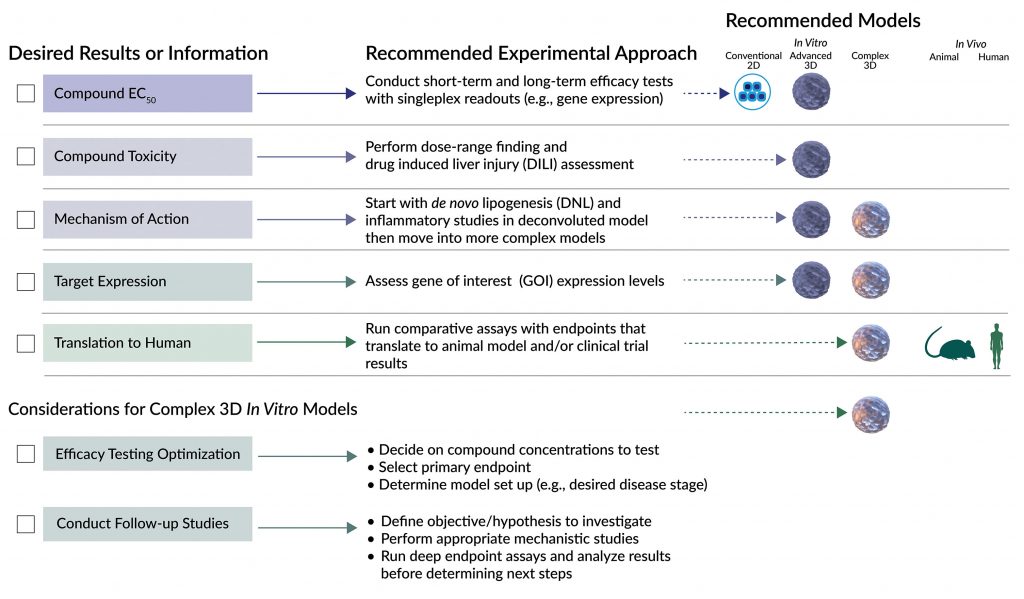
Using an antisteatotic drug candidate as an example, let’s take a closer look at experimental design considerations for selecting the most appropriate models for different types of experiments at various stages in the drug development pipeline.
Target-based drug discovery
If our antisteatotic compound of interest has already been identified in a target-based screen, we can retrieve basic information about its potency and specificity, such as whether it inhibits an enzyme involved in de novo lipogenesis (DNL). We will need to discern additional features of the drug, such as efficacy in hepatocytes, effect on lipid loading, and potential toxic effects.
For these types of efficacy and safety studies, we recommend the 3D InSight™ Human Liver Microtissues (Figure 3A). This advanced 3D co-culture model includes physiologically relevant cell types and a specially formulated cell culture medium with the high levels of glucose necessary for hepatocyte function in vitro and DNL pathway support. With this model, we can assess the efficacy of the drug by focusing on lipid loading as the functional output.
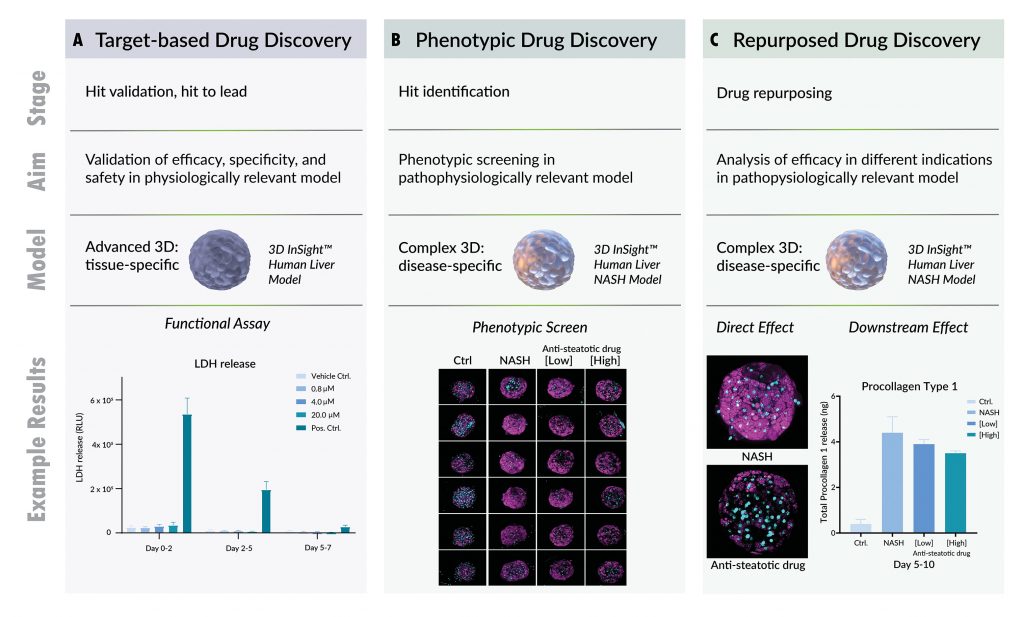
In parallel, multiplexed analysis of ATP levels and lactate dehydrogenase (LDH)leakage can yield additional details about cell viability, metabolism, and potential necrosis over long-term (7–14 days) exposure to the drug. Depending on the results of these studies, next steps could be analysis of secondary effects in a more complex NASH model or further refinement of concentrations and/or treatment schedule if the desired effect was not observed.
Phenotypic drug discovery
If we aim to identify novel antisteatotic drug candidates, we could conduct a phenotypic screening campaign to assess libraries of compounds for their ability to affect a disease-relevant feature, such as lipid loading, without focusing on a specific target. For phenotypic screening, model systems must replicate human physiology as closely as possible, while still allowing for reasonable throughput.
In this case, our 3D InSight™ Human Liver NASH model is applicable, as it enables screening of small- to medium-sized compound libraries with multiplexed endpoints in a pathophysiologically relevant system (Figure 3B). It provides a platform not only for assessing compound efficacy, but also useful parameters such as cell permeability, toxicity, and downstream effects. Ultimately, however, deconvolution of the mechanism of action and target identification must be performed. For these types of studies, we recommend a simpler model system to minimize confounding factors.
Repurposed therapeutics in drug discovery
If our antisteatotic compound has been repurposed from a different program/disease indication, we should have a wealth of useful data at our fingertips. Pharmacokinetic/pharmacodynamic (PK/PD) modeling, toxicity, and efficacy will have already been determined in vivo. But before we can advance the drug to the clinic for NASH trials, we will need information about how the compound will affect NASH-related pathways implicated in development of steatosis (as evidenced by lipid uptake, DNL, etc.). Some of these pathways may be differentially regulated in mice as compared to humans, and consequently, results from mouse studies may not translate to clinical findings.
We can analyze target expression and pathway activation in the 3D InSight™ Human Liver NASH model, because the same human donor cells are used to replicate healthy conditions and the induced disease state in vitro (Figure 3C). If experimental results show the pathway or target of interest is expressed/active in this model, previously recorded data on Cmax and EC50 can be used for a drug efficacy assessment. Such exploratory experiments enable efficient assessment of the primary and secondary effects of drug candidates on steatosis and, potentially, downstream inflammation and fibrosis.
Combinatorial drug discovery
The latest clinical trial results with NASH therapeutics suggest that successful NASH treatment will require a combination of different drugs. Because combinations are typically chosen to complement each other by targeting different pathways involved in NASH, the preclinical model used must represent each of these pathways. Although in vivo mouse models do have all required pathways, they are not a practical choice, simply because evaluating a full matrix of different drug combinations and concentrations would be prohibitively expensive. For initial screening of potential combinatorial approaches, we recommend a medium-throughput model such as the 3D InSight™ Human Liver NASH model, with one or two automation-compatible, multiplexed endpoints.
Adapting to rapidly evolving technology
Although no off-the-shelf kit or single model will provide deep mechanistic insight into complex diseases, such as NASH, tremendous advances in 3D cell technologies over the past few years have expanded the resources available to researchers involved in NASH drug discovery. We recommend researchers regularly review and adapt their experimental designs to fully exploit the potential of rapidly evolving technologies (3D cell culture platforms, readout technologies, automation, etc.) to better understand, and ultimately treat, complex diseases with high unmet medical needs.
Sue Grepper, PhD, is senior application scientist, Karen Dowell, PhD, is marketing manager, and Eva Christina Thoma, PhD ([email protected]), is head of the Liver Solutions Group at InSphero.