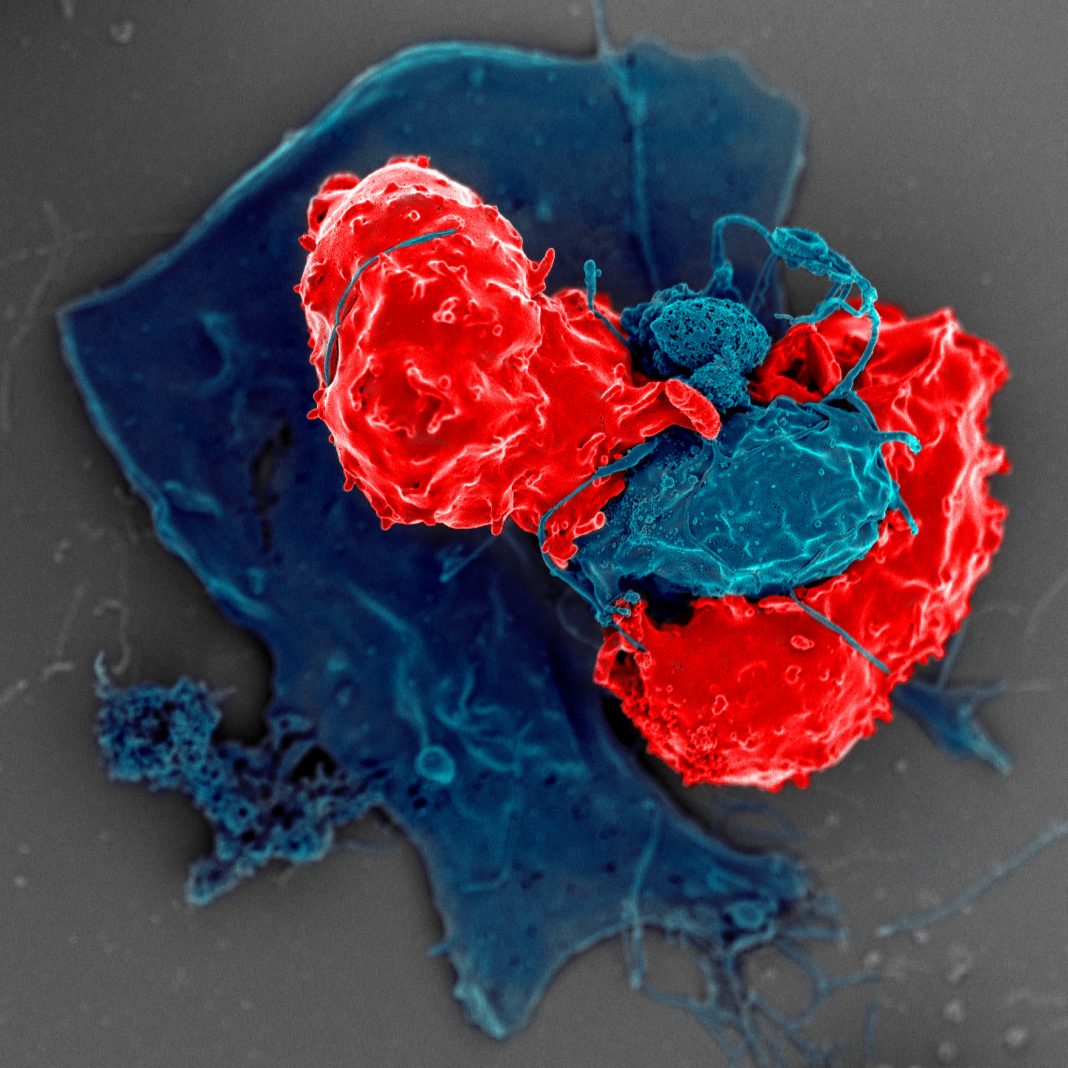
Cellular immunotherapy is emerging as a fifth pillar of cancer treatment. Clinical efficacy has been proven for a variety of cancers; however, its true potential has yet to be fully realized. The forerunner to the majority of cellular immunotherapeutics currently being developed are chimeric antigen receptor (CAR) T cells, and while new, distinct avenues have been pursued, CAR T cells have undergone several generations of improvement.
First-generation CAR T cells demonstrated that these cells, created by the advent of genetic engineering, could be designed for antigen-specific killing. Second- and third-generation products have become more potent due to their incorporation of costimulatory
domains for enhanced efficacy. Blueprints for future generations include programmed patterns of migration/tumor infiltration (via constitutive/deleted chemokine receptor expression), secretion of cytokines that oppose the immunosuppressive mandate of the tumor microenvironment, and means of limiting “exhaustion” (for example, T-cell production of anti-PD-1 or anti-PD-L1/2 antibodies).
CAR T-cell immunotherapeutics like Kymriah, the first cellular immunotherapeutic approved by the FDA, are not the only cellular immunotherapics. Although the activation of CAR T cells is dependent only upon the binding of antigen to its receptor, many emerging cellular immunotherapeutics, such as those incorporating T-cell receptor (TCR) technology, require major histocompatibility complex (MHC) restriction of antigen recognition.
In TCR immunotherapeutics, T cells are isolated from patients, TCR specificities are altered (via retroviral transduction), modified T cells are expanded (via IL-2/IL-7/IL-15), and finally, modified T cells are administered to the patient. This alternative approach may offer advantages, such as a reduced likelihood of inducing a potentially fatal “cytokine storm.”
Limiting excessive on-target effects segues into limiting off-target killing, that is, cytotoxic T lymphocyte (CTL)-mediated loss of healthy cells. Therefore, while the genetic engineering of cellular immunotherapeutics is primarily aimed at enhancing on-target efficacy, it also includes careful consideration of off-target killing.
Several innovative approaches are being tested to see if they can improve the safety profile without sacrificing potency. In one of these approaches, T cells are engineered to incorporate on/off logic gates that can collate local signals and inform whether—and to
what extent—activation should occur.
Screening protocols
Ultimately, empirical data is the only way to assess the impact of altered biology. The rapid expansion in high-concept genetic engineering has generated the need for a screening protocol that provides quantification of on-target killing while screening for off-target killing. At Charles River, we have designed a comprehensive and adaptable library of screening protocols which cover critical cell types of the human body. This platform enables the creators of cellular immunotherapeutics to initially screen for lead candidates then assess unwanted profiles in support of their application for clinical trials. Here we describe, in broad terms, the basis of this novel assay.
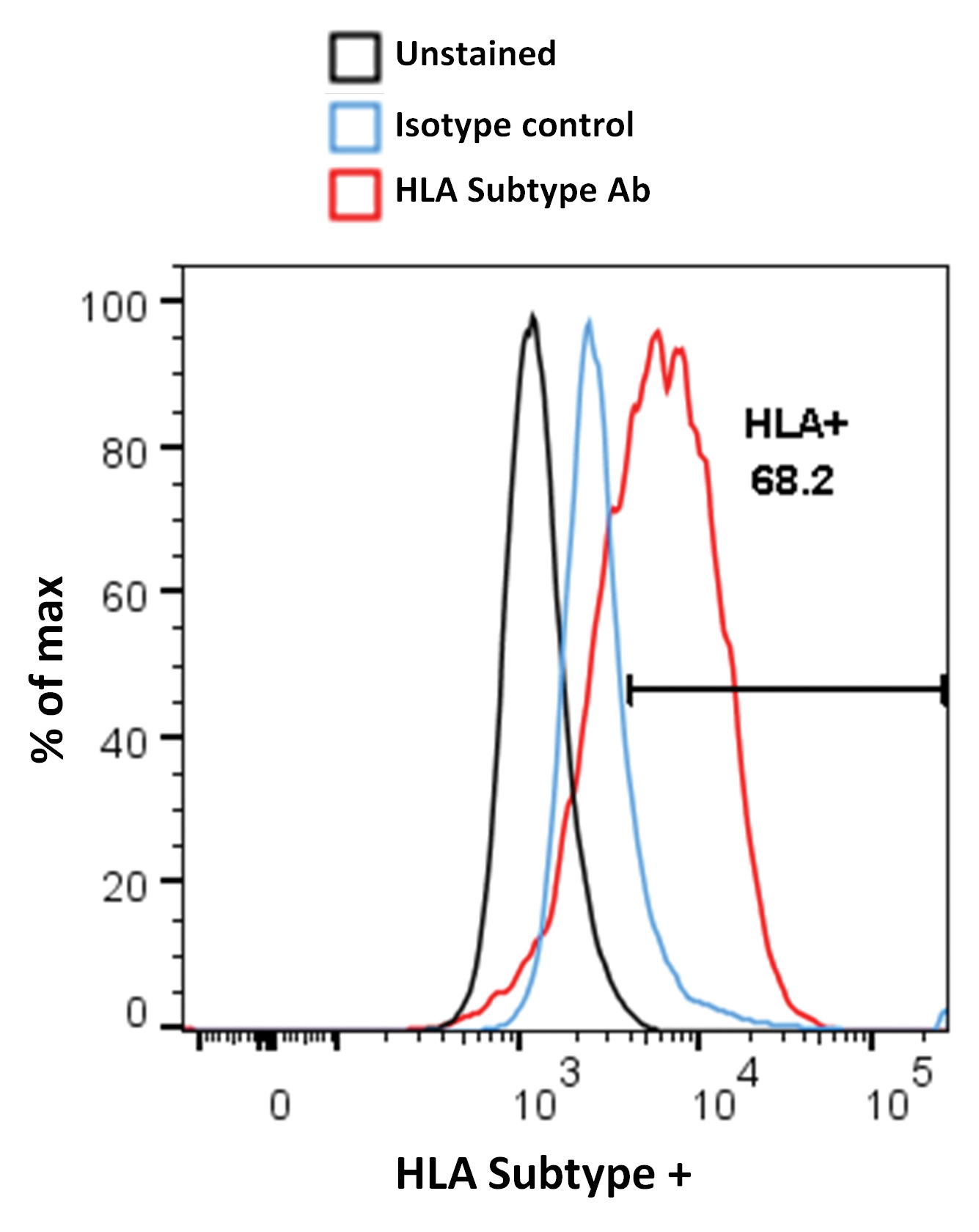
The first step is to identify human “Target” cells that express the human leukocyte antigen (HLA) subtype capable of presenting the tumor antigen in a form the cellular therapeutic can recognize. To achieve this, we screen primary and induced pluripotent stem cell (iPSC)-derived human cells for HLA subtype expression by flow cytometry (FC) (Figure 1). Once suitable donors have been identified, these cells are loaded with a preparation of the relevant tumor peptide.
“Effector” cells (constituting the cellular immunotherapeutic) are then included in co-culture experiments across a range of Target:Effector cell ratios. On-target killing is quantified as induction of Target cell apoptosis in conjunction with Effector cell activation. Effector cell activation may be quantified via cytokine analysis by time-resolved fluorescence resonance energy transfer (TR-FRET) and/or activation marker expression (by FC). For non-adherent Target cells, the Effector cells are labeled prior to their addition to allow their exclusion from downstream flow cytometric analysis of viability. Off-target effects are assessed by performing parallel experiments where the target cells are not pulsed with antigen.
For adherent cell types, a live-cell imaging approach is taken using systems such as the IncuCyte system. This approach has several advantages, including the following:
1. Cells are not detached from the surface—an event that may itself induce the death of more fragile cell types, such as neurons.
2. Target cell numbers can be orders of magnitude lower than those required for FC—an important consideration when the Target cells may be valuable/difficult to obtain and culture.
3. This approach is data-rich, as read-outs are collected on the hour, every hour, throughout the course of the experiment.
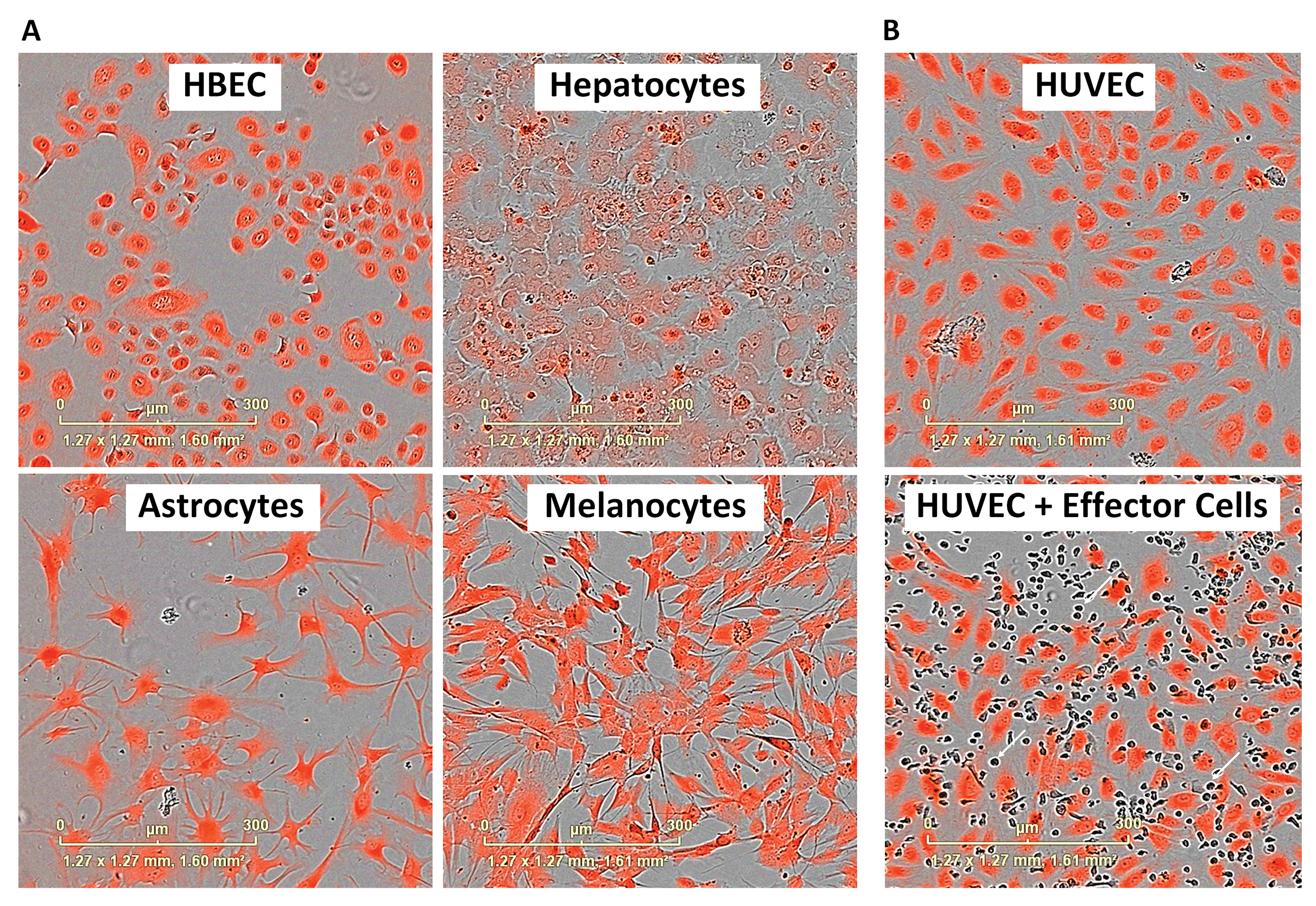
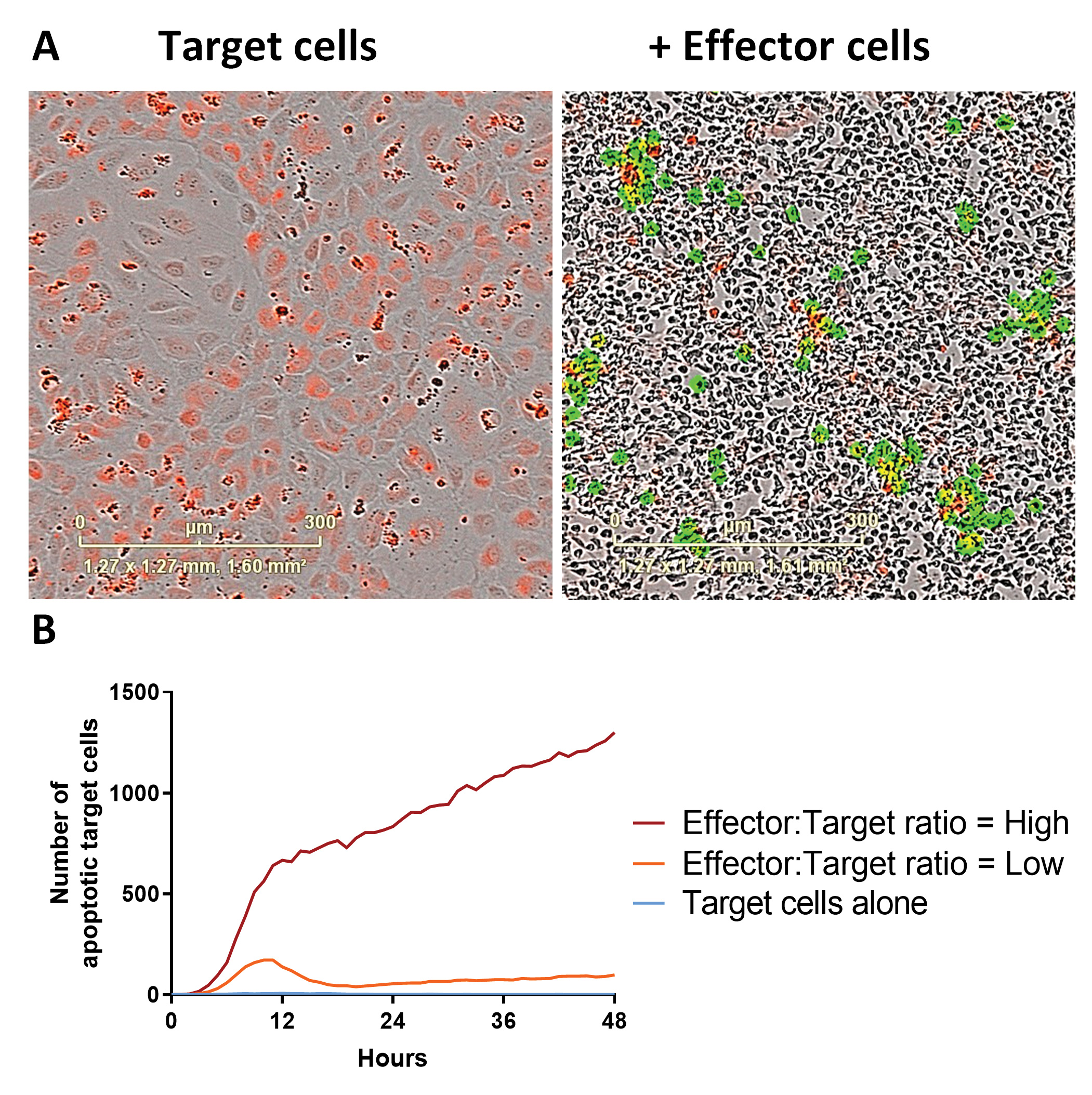
We label the Target cells with a dye suitable for the live-cell imaging system, which may include CytoLight (Figure 2) or nuclear red fluorescent protein (genes for which may be inserted via transient lentiviral transduction). An apoptotic live-read marker is included in the media of the co-culture experiment (for example, caspase-3/7 or annexin V). By colocalizing the apoptotic marker with the Target cell, we can track Target cell apoptosis over time (Figure 3). A suitable cytotoxic control is included for comparison.
Assay parameters
This approach to screening off-target effects of cellular immunotherapeutics is not without its challenges. These mainly arise from the need to optimize the experimental parameters for the requirements/characteristics of what may be very different cell types.
For example, neurons are difficult to obtain, maintain, and label. As they express few MHC class I molecules, the kinetics of their killing can be prolonged as CTL activation is low. By contrast, under comparable Target:Effector ratios, some primary cells are rapidly killed (in some cases, within 5 minutes). Where cells prefer to exist as a confluent monolayer, such as hepatocytes, the creation of an image analysis mask capable of defining individual Target cells is not trivial.
The cell labeling choice is crucial and cell line dependent. CytoLight dye is often taken up efficiently, but the intensity of the signal diminishes over time. By contrast, epithelial cells maintain a robust CytoLight signal longer. Fortunately, as apoptotic cells condense, the dye becomes brighter, enabling the technique to work for the majority of cell types. Where cell dye is retained, a secondary readout can be utilized.
Loss of total Target cell area can be used to confirm the extent of killing in parallel to “target specific apoptotic cell death.” Relatedly, experienced operators are necessary as Target cell apoptosis may be overestimated if punctate staining of the target cells combines with multiple Effector cells acting on a single Target cell at different locations. These, and other hurdles, can be overcome by fine-tuning the parameters of both the assay set-up and the imaging mask used for analysis.
Conclusion
Our capacity to offer a screening panel representative of the differing cell types of the human body is based on expertise developed through years of immuno-oncology experience. These techniques can be used across a wide range of validated assays, and this may be expanded as required. This platform represents an opportunity to accelerate the development of cellular immunotherapeutics, such as those based on CAR T cells, TCRs, or CAR natural killer cells, that show both higher potency and improved safety.
Jezrom Self-Fordham, PhD, is manager, immunology, and Robert Nunan, PhD, is group leader, cell biology, at Charles River Laboratories.