Tarantulas, the well-known hairy and scary spiders, are the world’s largest spiders. Most tarantulas are busy minding their own business and won’t bother you if you don’t bother them. However, one type of tarantula, the Chinese bird spider, has reason to be feared. The venom of the Chinese bird spider is a neurotoxin that causes severe nerve damage, rendering the victim incapable of moving, and sometimes causing death if not treated. Now, researchers from the University of Washington Health Sciences, have captured this information and added it to their web of knowledge. The researchers report the toxin may hold answers to better control of chronic pain.
Their findings are published in the journal Molecular Cell in a paper titled “Structural Basis for High-Affinity Trapping of the NaV1.7 Channel in Its Resting State by Tarantula Toxin.”
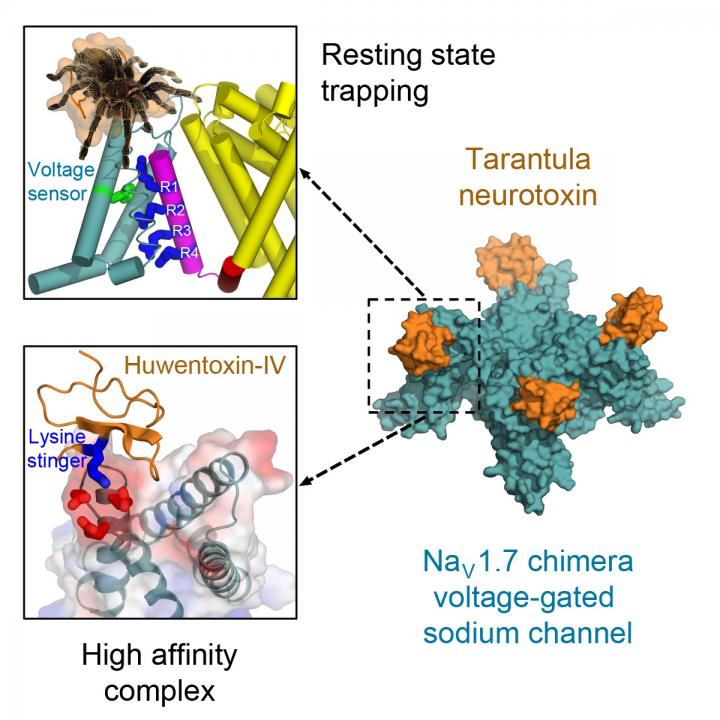
Using a high-resolution cryo-electron microscope, researchers were able to show how the stinger locks the voltage sensors on sodium channels. Trapped in their resting position, the voltage sensors are unable to activate.
Sodium channels play a role in the maintenance of inflammatory pain states. These channels have a critical role in the development and maintenance of several pain syndromes, including inflammatory pain, neuropathic pain, and central pain associated with spinal cord injury.
“The action of the toxin has to be immediate because the tarantula has to immobilize its prey before it takes off,” explained William Catterall, professor of pharmacology at the University of Washington School of Medicine. He was the senior researcher, along with pharmacology professor and Howard Hughes Medical Institute investigator, Ning Zheng, on the study of the molecular damage inflicted by tarantula venom.
Such studies of toxins from these “big, nasty dudes,” as Catterall describes them, could point to new approaches to structurally designing drugs that might treat chronic pain by blocking sensory nerve signals.
Catterall explained that chronic pain is a difficult-to-treat disorder. Efforts to seek relief can lead to opiate overdose, addiction, prolonged withdrawal, and even death. The development of safer, more effective, non-addictive drugs for pain management is a vital need.
“The structural basis for tarantula-toxin action remains elusive because of the difficulty of capturing the functionally relevant form of the toxin-channel complex. Here, we engineered the model sodium channel NaVAb with voltage-shifting mutations and the toxin-binding site of human NaV1.7, an attractive pain target,” noted the researchers.
The researchers took the toxin-binding region and imported it into their model ancestral sodium channel from a bacterium.
“This mutant chimera enabled us to determine the cryoelectron microscopy (cryo-EM) structure of the channel functionally arrested by tarantula toxin. Our structure reveals a high-affinity resting-state-specific toxin-channel interaction between a key lysine residue that serves as a ‘stinger’ and penetrates a triad of carboxyl groups in the S3-S4 linker of the voltage sensor,” the researchers wrote.
Their achievement revealed the structural basis for voltage sensor trapping of the resting state of the sodium channel by this toxin.
“Remarkably, the toxin plunges a ‘stinger’ lysine residue into a cluster of negative charges in the voltage sensor to lock it in place and prevent its function,” Catterall said. “Related toxins from a wide range of spiders and other arthropod species use this molecular mechanism to immobilize and kill their prey.”