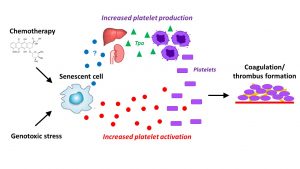
Flawed cells may enter senescence, or cell cycle arrest, to avoid passing on their flaws, but they can still cause trouble. Accumulating year by year and staying metabolically active while secreting harmful factors, senescent cells contribute to various age-related diseases. So far, the factors that contribute to inflammation have attracted the most attention. But a new set of factors has been uncovered by researchers at the Buck Institute. These factors appear to participate in hemostasis.
It is probably no coincidence, then, that risks for certain hemostasis-related disorders, such as thrombosis, increase with age. Another intriguing correlation exists, various research teams have noted, between the administration of cancer drugs, which damage DNA and induce cellular senescence, and an increased risk of developing blood clots.
Despite these correlations, scientists have hesitated to cite a link between cellular senescence and thrombosis. Before positing such a link, scientists would prefer to have supporting evidence—evidence of the sort that has been sought, and found, by a Buck Institute team led by Judith Campisi, PhD, a professor of biogerontology.
Using stable isotope labeling with amino acids (SILACs) and cultured cells, the team identified 343 proteins that senescent human fibroblasts secrete at twofold or higher levels compared with quiescent counterparts, that is, cells that reversibly suspend replication. After performing bioinformatic analyses, the scientist determined that 44 of these proteins participate in hemostasis.
Additional details, including evidence that some of the proteins occur in mice, appeared September 24 in the journal Cell Reports, in an article titled, “SILAC Analysis Reveals Increased Secretion of Hemostasis-Related Factors by Senescent Cells.” This article also presented findings reinforcing the idea that chemotherapy-induced senescence could potentiate blood clotting, most likely by sensitizing platelets to agonists rather than activation of the coagulation cascade.
“Mice treated with the chemotherapeutic agent doxorubicin, which induces widespread cellular senescence in vivo, show increased blood clotting,” the article’s authors wrote. “Conversely, selective removal of senescent cells using transgenic p16-3MR mice showed that clearing senescent cells attenuates the increased clotting caused by doxorubicin.”
Basically, the mice showed increased blood clotting, similar to what happens in humans who undergo chemotherapy. “Conversely, when we selectively removed senescent cells in specially bred transgenic mice, the increased clotting caused by doxorubicin went away,” said Christopher Wiley, PhD, an assistant research professor at the Buck Institute and the lead author of the paper.
“Some people throw up their hands and say senescence is too complicated to make sense of,” noted Campisi, the article’s senior author. “But this paper shows that we can identify what’s going on.” In addition, the paper indicates that in recipients of chemotherapy, adjuvant therapies that suppress the senescence-associated secretory phenotype (SASP) might reduce the side effect of enhanced platelet activation. Information about SASP components, Campisi suggested, could “be useful in targeting who gets treatment and when they should get it.”
“The incidence of venous thrombosis, which includes deep vein thrombosis and pulmonary embolism is extremely low until the age of 45, when it begins to rise rapidly. Over time, it becomes a major risk factor for death. By 80, the condition affects five to six people per thousand individuals,” Campisi elaborated. “Blood clots are also a serious side effect of chemotherapy, which sets off a cascade of senescence in those undergoing treatment. That’s why blood thinners, which carry their own risks, are often included in treatment protocols.”
Scientists in the Campisi lab and other labs around the world are working to develop senolytics, drugs that would clear senescent cells from the body, potentially providing treatment options for many age-related diseases that are either caused or linked to senescence. They include Alzheimer’s and Parkinson’s diseases, cardiovascular disease, osteoarthritis, macular degeneration, age-related cancers, and sarcopenia, among others.