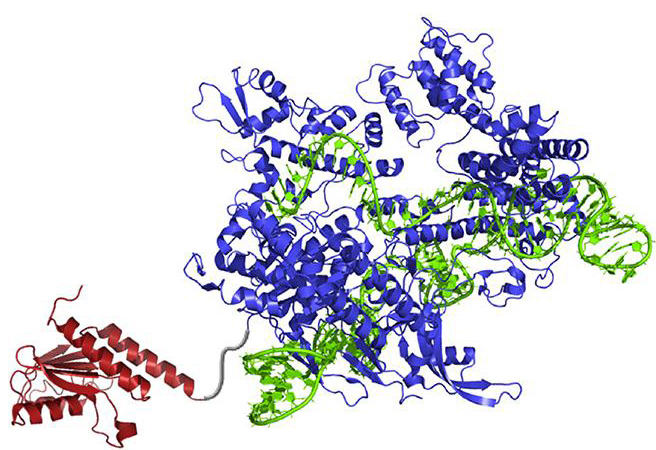
A new enzyme, called an adenine base editor (ABE), can convert selected A-T base pairs to G-C base pairs, and do so without cleaving DNA. ABE’s capabilities may be attributed to its main components: an “evolved” transfer RNA adenosine deamininase (TadA), a Cas9 nickase, and a guide RNA.
ABE can directly repair the type of single-letter changes in the human genome that account for approximately half of human disease-associated point mutations. These mutations are associated with disorders ranging from genetic blindness to sickle cell anemia to metabolic disorders to cystic fibrosis.
ABE actually refers to a family of base editors, the members of which differ because they represent different evolved versions of the TadA enzyme. TadA is the starting point for ABEs because it is able to convert adenine to inosine, which cells treat as guanine. Ordinary or natural TadA, however, works only on transfer RNA, not DNA. To create DNA-modifying versions of TadA, scientists based at Harvard University and the Broad Institute of MIT generated TadA mutants in bacterial cells.
These bacterial cells were required to convert adenine to inosine in antibiotic resistance genes in order to survive antibiotic challenge. Surviving bacteria encoded TadA mutations that imparted the ability to perform the adenine-to-inosine conversion on DNA.
To assemble an ABE, the Harvard/Broad team attach a mutated TadA to a modified form of the Cas9 enzyme. Ordinarily, as part of a CRISPR/Cas9 gene-editing system, Cas9 will cleave DNA, which is then repaired by one or another set of molecular machinery residing in the living cell. The Cas9 used in ABE, however, is modified so that it only nicks DNA at the base opposite the adenine-to-inosine conversion site. The nick prompts the cell to insert the correct partner base pair to match the new one, thereby completing the swap of A-T to G-C.
Details about the ABEs appeared October 25 in the journal Nature, in an article entitled “Programmable Base Editing of A•T to G•C in Genomic DNA without DNA Cleavage.” The article’s senior author, David Liu, Ph.D., led an effort last year to create a base editor that could convert C-G base pairs to A-T base pairs. Now Dr. Liu and colleagues introduce another tool, one that can accomplish the opposite function.
“Extensive directed evolution and protein engineering resulted in seventh-generation ABEs (e.g., ABE7.10), that convert target A•T to G•C base pairs efficiently (~50% in human cells) with very high product purity (typically ≥ 99.9%) and very low rates of indels (typically ≤ 0.1%),” wrote the article’s authors. “ABEs introduce point mutations more efficiently and cleanly than a current Cas9 nuclease-based method, induce less off-target genome modification than Cas9, and can install disease-correcting or disease-suppressing mutations in human cells.”
Experiments in the new study hint at the promise of the new ABEs. ABE7.10 reversed a G-to-A mutation associated with a genetic iron-storage disease known as hemochromatosis in cells taken from patients. In a different experiment, ABE7.10 added a mutation that restored the function of a hemoglobin gene in human cells. That mutation is known to confer protection against blood diseases including sickle cell anemia.
“We are hard at work trying to translate base-editing technology into human therapeutics,” said Liu, but many hurdles remain. Safety, efficiency, and base editor delivery methods still need to be answered before base editing can be used to tweak the human genome. “Having a machine that can make the change you want to make is only the start,” Liu added. “You still need to do all this other work, but having the machine really helps.”
The new system is a “really exciting addition to the genome engineering toolbox,” commented Feng Zhang, Ph.D., an HHMI-Simons Faculty Scholar and molecular biologist at the Broad Institute of MIT and Harvard, who was not involved in the study. (Zhang led an effort that culminated in a new RNA-editing tool.) “It's a great example of how we can harness natural enzymes and processes to accelerate scientific research.”