An international research team has described a potential new approach to holding back the loss of cognitive function that occurs with Alzheimer’s disease (AD), which uses a synthetic compound to boost protein synthesis in neurons. The scientists’ studies in mouse models of the neurodegenerative disorder demonstrated how the experimental small molecule could rescue the activity of brain cells needed for memory formation. “This work is the first to show that reversing impaired protein synthesis in brains afflicted by Alzheimer’s disease through a pharmacological approach is not only feasible, but also effective,” commented Mauricio Martins-Oliveira, PhD, a postdoctoral researcher at New York University’s Center for Neural Science, and first author of the team’s paper in Science Signaling, which is titled, “Correction of eIF2-dependent defects in brain protein synthesis, synaptic plasticity, and memory in mouse models of Alzheimer’s disease.”
Alzheimer’s disease (AD) is the most prevalent form of dementia, and affects more than 35 million people worldwide, the authors stated. Pathological characteristics of the disease include the accumulation of amyloid-β (Aβ) peptide in the brain, and the formation of neurofibrillary tangles composed of hyperphosphorylated tau protein, neuroinflammation, synapse failure/loss, and neurodegeneration, which ultimately culminate in memory failure. Current treatments are centered on reducing certain features of the disease, such as amyloid plaque load, neurofibrillary tangles, and neuroinflammation.
And while there has been significant progress in understanding some of the pathogenic mechanisms, the team continued, there is still an urgent need for effective therapeutics that can prevent or block the progressive cognitive deterioration. “Given the complex nature of AD, identification of molecular pathways and targets that effectively improve cognition has been challenging.”
The synthesis of new proteins in the brain is essential for proper neuronal function and, notably, for memory consolidation. Neuronal protein synthesis is essential for long-term memory consolidation, and its dysregulation is implicated in AD, and in other neurodegenerative disorders, the team continued. “De novo protein synthesis plays a key role in synaptic plasticity and long-term memory consolidation.” But cellular stresses can triggers activation of eIF2α protein kinases, which cause phosphorylation of eIF2α in a process known as the integrated stress response (ISR). And this ISR attenuates protein synthesis.
Interestingly, mounting evidence has implicated aberrant ISR and eIF2α-P in various brain disorders, including traumatic brain injury, prion disease, Down syndrome, and AD, the team continued. “ISR markers are increased in AD patient brains, as well as in the brains of mouse models of AD.”
Co-senior study author Sergio Ferreira, PhD, a professor at the Institute of Biophysics at Brazil’s Federal University of Rio de Janeiro (UFRJ), further explained, “We and others have previously shown that impairments in brain protein synthesis contribute memory deficits in Alzheimer’s disease model mice, and that the brains of Alzheimer’s patients exhibit clear signs of impaired protein synthesis. We thus asked ourselves whether rescuing brain protein synthesis might be an approach to improve memory function in Alzheimer’s disease. As the authors further commented, “We previously demonstrated that either genetic ablation or inhibition of eIF2α kinases rescues synapse function and memory defects in AD mouse models.” They hypothesized whether rescuing brain protein synthesis downstream of eIF2α-P might be “an attractive approach” to correct cognitive impairment in AD.”
The team’s study centered on ISRIB, a synthetic molecule capable of boosting protein synthesis. Developed by Peter Walter at the University of California, San Francisco, ISRIB is an ISR inhibitor that specifically targets the process of translation initiation, ultimately stimulating cellular protein synthesis. The NYU and UFRJ researchers studies aimed to determine if ISRIB could thus work to restore synaptic plasticity—the ability of the brain to change in order to learn—and memory.
The investigators identified increased eIF2α phosphorylation, and reduced levels of eIF2B— another key component of the translation initiation complex—in post-mortem brain tissue from AD patients. After establishing that the components of protein synthesis machinery were depleted in the hippocampus—part of the brain structure known to play a significant role in memory—the scientists concluded that protein synthesis may also be impaired.
ISRIB stimulates eIF2B activity, even in the presence of increased eIF2α, so the team tested whether ISRIB could rescue memory in one mouse model of AD, which they assessed through a series of well established memory tests (such as navigating a maze). The results confirmed that treatment using ISRIB did lead to improved memory functions in these mice, as well as restore protein synthesis in the hippocampus.
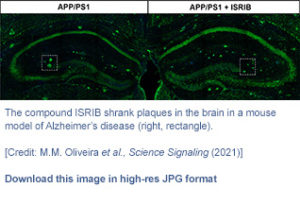
Taken together, the researchers’ findings indicated that restoring protein synthesis, aided by the synthetic molecule ISRIB, could work to restore cognitive processes impaired by Alzheimer’s disease. “In conclusion, our findings establish eIF2α-P–dependent translational repression as a potentially druggable target in AD and reveal unexplored avenues to investigate the molecular mechanisms by which dysfunctional eIF2 signaling contributes to translational repression in AD,” they wrote. “… attenuating the ISR downstream of phosphorylated eIF2α may restore hippocampal protein synthesis and delay cognitive decline in AD patients.”
“Given the complex nature of Alzheimer’s disease, identifying and targeting abnormal molecular pathways that effectively improve cognition has been challenging,” commented co-senior author Eric Klann, PhD, a professor in NYU’s Center for Neural Science. “Our findings show that jump-starting protein synthesis in the brain can revive lost cognitive functions. We hope that this work can serve as a step forward in treating this devastating disease.”






