“A little dab’ll do ya!” That was the Brylcreem slogan for their hair product some 60 years ago. Now, it seems, that researchers at Northwestern University are applying the same motto to mitochondrial stress and cellular health. In a genetic study of the roundworm Caenorhabditis elegans, the investigators found that signals from mildly stressed mitochondria prevent the failure of protein-folding quality-control (proteostasis) machinery in the cytoplasm that comes with age. Findings from the new study—published recently in Cell Reports, in an article entitled “Mitochondrial Stress Restores the Heat Shock Response and Prevents Proteostasis Collapse during Aging”—could help researchers better understand the molecular mechanisms that drive aging and risk for age-associated degenerative diseases.
Additionally, the research team noted that the stabilized proteostasis mechanisms from moderately stressed mitochondria suppressed the accumulation of damaged proteins that can occur in degenerative diseases, such as Alzheimer’s, Huntington’s, and Parkinson’s diseases and amyotrophic lateral sclerosis (ALS).
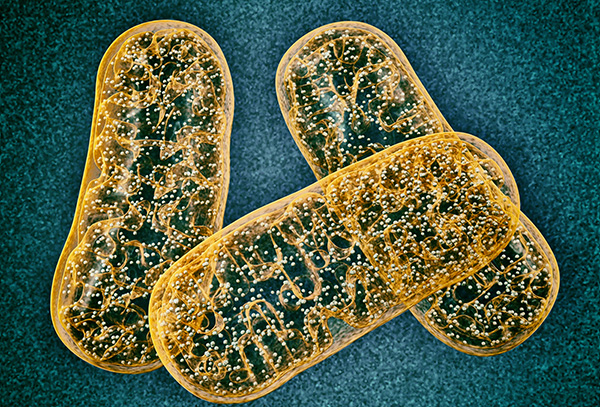
![In this photograph of cow cells, the mitochondria were stained in bright yellow to visualize them in the cell. The large blue dots are the cell nuclei and the gray web is the cytoskeleton of the cells. [NIH]](https://genengnews.com/wp-content/uploads/2018/08/29700957935_274d15f45b_z2511371711-1.jpg)
In this photograph of cow cells, the mitochondria were stained in bright yellow to visualize them in the cell. The large blue dots are the cell nuclei and the gray web is the cytoskeleton of the cells. [NIH]
“This has not been seen before,” said senior study investigator Richard Morimoto, Ph.D., a professor in the department of molecular biosciences at Northwestern. “People have always known that prolonged mitochondrial stress can be deleterious. But we discovered that when you stress mitochondria just a little, the mitochondrial stress signal is actually interpreted by the cell and animal as a survival strategy. It makes the animals completely stress resistant and doubles their lifespan. It’s like magic.”
C. elegans may seem like an unlikely model, but the roundworms have a biochemical environment and cellular properties similar to that of humans—making them a popular research tool for studying the biology of aging and as a model of human disease. The components identified by the Northwestern team as playing a role in biological aging are conserved in all animals, including humans, and offer targets for future study.
“Our findings offer us a strategy for looking at aging in humans and how we might prevent or stabilize against molecular decline as we age,” Morimoto said. “Our goal is not trying to find ways to make people live longer but rather to increase health at the cellular and molecular levels so that a person’s span of good health matches their lifespan.”
![Using the nematode <i>C. elegans</i>, Labbadia et al. demonstrate that low levels of mitochondrial stress caused by exposure to RNA interference (RNAi) or xenobiotics can restore HSF-1 function with age, thereby maintaining cytosolic proteostasis, enhancing stress resistance, and prolonging healthspan, all without detrimental effects on development or reproduction. [Labbadia et al., 2017, Cell Reports 21, 1481–1494 November 7, 2017]” /><br />
<span class=](https://genengnews.com/wp-content/uploads/2018/08/fx17021223866.jpg) Using the nematode C. elegans, Labbadia et al. demonstrate that low levels of mitochondrial stress caused by exposure to RNA interference (RNAi) or xenobiotics can restore HSF-1 function with age, thereby maintaining cytosolic proteostasis, enhancing stress resistance, and prolonging healthspan, all without detrimental effects on development or reproduction. [Labbadia et al., 2017, Cell Reports 21, 1481–1494 November 7, 2017]
Using the nematode C. elegans, Labbadia et al. demonstrate that low levels of mitochondrial stress caused by exposure to RNA interference (RNAi) or xenobiotics can restore HSF-1 function with age, thereby maintaining cytosolic proteostasis, enhancing stress resistance, and prolonging healthspan, all without detrimental effects on development or reproduction. [Labbadia et al., 2017, Cell Reports 21, 1481–1494 November 7, 2017]
The current study builds upon on a 2015 study by the same authors in which they reported that the molecular decline leading to aging begins at reproductive maturity due to inhibitory signals from the germline cells to other tissues to prevent induction of protective cell stress responses. In C. elegans, this is between 8 and 12 hours of adulthood, yet the animal will typically live another three weeks. Knowing that the molecular health of C. elegans fails between day one and day two of adulthood, in their current study the researchers looked at two-day-old animals to identify genes and pathways that would prevent the molecular failure.
“To identify the factors driving this event, we performed an unbiased genetic screen for suppressors of stress resistance and identified the mitochondrial electron transport chain (ETC) as a central regulator of the age-related decline of the HSR [heat shock response] and cytosolic proteostasis,” the authors wrote. “Mild downregulation of ETC activity, either by genetic modulation or exposure to mitochondria-targeted xenobiotics, maintained the HSR in adulthood by increasing HSF-1 binding and RNA polymerase II recruitment at HSF-1 [heat shock factor 1] target genes. This resulted in a robust restoration of cytoplasmic proteostasis and increased vitality later in life, without detrimental effects on fecundity.”
“I never would have guessed this—a low-stress signal resets the organismal lifespan profoundly,” Dr. Morimoto concluded. “What we are learning is that some of these stress signals are interpreted by the organism as a way to reset itself and to live longer. When mitochondria function optimally, the cells and tissue are robust.”