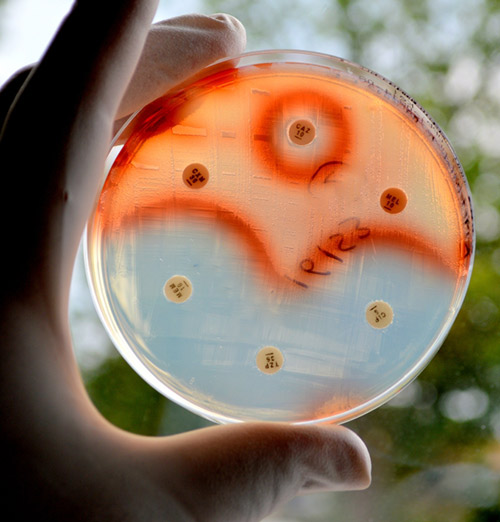
Notorious, antibiotic-resistant bacteria that form biofilms, such as Pseudomonas aeruginosa, can evolve to survive antimicrobial nanosilver treatment. New research from scientists at the University of Technology Sydney (UTS) demonstrates long-term nanosilver treatment can increase the risk of recurrent infections of such pathogenic strains.
The scientists explore the long-term response and adaptation of P. aeruginosa biofilms to nanosilver—silver particles about two nanometers in diameter held on inert titanium support, and compare this to its response to ionic silver—silver nitrate salt, and the model antibiotic, gentamycin, in contrast to free-living or planktonic forms of the bacteria. They show, while the potent antimicrobial silver agents effectively inhibit the growth of P. aeruginosa biofilms, prolonged exposures to these agents cause the bacteria to evolve persistence.
The new study that highlights the phenomenon of nanoparticle adaptation of bacterial biofilms is published in an article in the Journal of Nanobiotechnology titled, “Evolution of bioflm-forming pathogenic bacteria in the presence of nanoparticles and antibiotic: adaptation phenomena and cross-resistance.”
“Understanding how pathogens develop adaptation mechanisms to nanoparticles is key in our effort to overcome the phenomena, including in biofilms as the major form of growth of pathogenic bacteria. This is to protect the efficacy of important alternative antimicrobials, like nanosilver, in this era of increasing antibiotic resistance,” said Cindy Gunawan, PhD, corresponding author on the paper.
Riti Mann, PhD, first-author of the paper says the study will help develop better management strategies for the use of nanoparticles as antimicrobials, particularly for long-term treatments. Mann says, “Based on this study, we recommend monitoring patients not only during but also after prolonged use of nanoparticle treatment for safeguarding against recurrent infections.”
More than half of all chronic infections, including hospital-related infections, arise due to the growth of stable bacterial biofilms that attach to the surfaces of wounds, ventilators, intravenous and urinary catheters, cardiac pacemakers, and other medical devices and prostheses. Hospital-related infections in the US alone incur a healthcare cost of over $94 billion annually.
Bacterial biofilms are more resilient to antimicrobial treatments than free-living bacteria since they produce a protective polymeric matrix that reduces the penetration of antibiotics to the bacteria. And it’s not just that. Bacteria in biofilms can synthesize signaling molecules (auto-inducers) that activate virulence genes, toxins, and protective matrix components, and transfer antibiotic resistance genes from cell to cell at a higher frequency. Together, these safeguard the microbial community in biofilms from antibacterial treatments.
The confounding challenges of resistant bacterial biofilms have spurred research on non-antibiotic options to inhibit and eradicate biofilm growth. Silver nanoparticles (nanosilver, NAg) have emerged as a potent antimicrobial and are used not only to fight or prevent infections in wound dressings and medical devices, but are also used in consumer products such as soaps, toothpaste, washing machines, refrigerators, and even in ‘odorless’ socks for kids.
In this study, researchers at UTS’s iThree Institute show the silver treatment does not result in the bacteria developing resistance. When a higher dosage of the antibacterial agent is required to inhibit bacterial growth, the bacteria is said to have developed ‘resistance’ against the agent, but when it takes longer for the antibacterial agent to kill the population, the bacteria is said to have developed ‘tolerance’ or ‘persistence’.
Whereas ‘tolerance’ indicates a bacterial population can survive longer in the presence of the antimicrobial agent due to a slower killing rate, ‘persistence’ in addition, indicates only a fraction of bacterial cells, called ‘persisters’, survive exposure to an otherwise lethal concentration of the antibacterial agent.
In this study, the scientists show nanosilver kills 99.99% P. aeruginosa in a sample population with only 0.01% of cells persisting. This minute fraction of ‘persister cells’ however, resume normal growth once the nanoparticle treatment is stopped.
Gunawan says, “The scientific evidence that bacteria can adapt to nanoparticles means we need effective regulation of the use of nanoparticles, with clear risks versus benefits assessment and clear antimicrobial targets. With limited development of new effective antibiotics over the past decades, we need to preserve the efficacy of the alternative antimicrobials to fight untreatable infections, saving lives and billions of dollars in healthcare.”