Scientists in Switzerland have identified differences in how brain circuitry connects to decision-making areas of the reward system in rodents with compulsive behaviors. Studies by the University of Geneva (UNIGE) researchers showed how decreasing the activity of a particular neural circuit in compulsive animals allowed them to gain more control of their behaviors, while stimulating the same connection in animals that otherwise demonstrated control, converted their behavior to addictive.
The studies, reported in Nature, shed light on the differences in brain function between people who are drug addicts, and those who are able to moderate their usage. “We do not know why one person becomes addicted to drugs while another doesn’t,” explained research lead Christian Lüscher, Ph.D., a professor at the departments of basic and clinical neurosciences of the UNIGE Faculty of Medicine. “But our study identifies the difference in brain function between the two behaviors.” The authors described their studies in a paper titled, “Stochastic synaptic plasticity underlying compulsion in a model of addiction.”
All addictive drugs target the mesolimbic dopamine system, and either directly or indirectly activate neurons that release dopamine, the authors explained. Addiction develops in a series of stages that begins with initial exposure, and moves initially on to controlled usage. Some people can keep control of their consumption, whereas others will progress to compulsive drug use, even though this will likely have a major adverse effect on aspects of their lives including their relationships, mounting debt, or even the risk of prison. Estimates suggest that about 1 in 5 people who use drugs will become addicted. “About 20% of users of addictive substances such as cocaine, heroin, and amphetamines eventually fulfill this diagnostic criterion,” the team wrote.
To try and understand how neural circuitry may impact on addictive behaviors, the researchers used an optogenetics approach to mimic how drugs of abuse activate the brain’s dopamine systems. They implanted an optic fiber into genetically engineered mice, which allowed the animal to stimulate its own reward system, located at the top of the brainstem. When the animal pressed a small lever, it switched on the fiber’s laser light, which activated dopamine neurons in the ventral tegmental area (VTA) of the brain. These are the same nerve cells that are activated by addictive drugs, and which are responsible for behavioral reinforcement.
After two weeks of daily test periods during which the mice repeatedly stimulated the neurons, the team then introduced a negative consequence, in the form of a brief electric shock administered to their feet, a third of the time that they pressed the lever, but at random. “We then introduce a weak electric shock so that we could observe, which mice continued with the self-stimulation,” explained Vincent Pascoli, Ph.D., lead author and researcher in UNIGE’s department of basic neurosciences. “This allowed us to identify the mice that had become compulsive.” They found that while 40% of the animals quickly stopped activating the lever following the introduction of the electric shock punishment— these mice were termed renouncers—60% of the animals, the perseverers, continued to stimulate their reward system, even though they also had to endure the painful foot shocks.
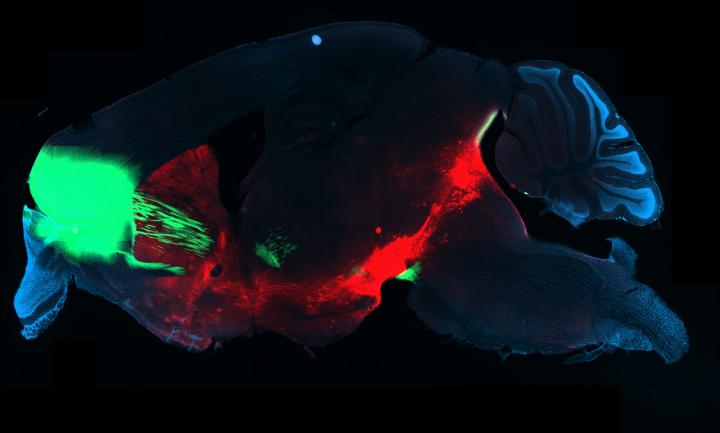
To try and determine the difference between the two groups of mice the team measured neuronal activity in different brain areas in real time. They found that communication between the orbitofrontal cortex (OFC), an area involved in decision-making, and the dorsal striatum, which is engaged in voluntary action, increased before lever-pressing in mice that were willing to obtain shocks along with dopamine self-stimulation.
“When we used an optical technique to visualize the activity in the brain of freely moving mice,” Dr. Lüscher commented. “This uncovered a stronger activity in a circuit of addicted mice compared to mice that did not lose control. The circuit extends from the orbito-frontal cortex to the dorsal striatum, a part of the basal ganglia of the brain.”
Interestingly, continuing compulsive behavior could be curbed in perseverers. Optogenetic inhibition of the identified neural pathway was enough to turn perseverers back into renouncing mice, although the effects were only temporary. When optogenetic inhibition was switched off the compulsive behavior returned. Similarly, artificially stimulating activity of the neural circuit in mice that were otherwise in control of their lever-pressing compulsion triggered the animals to develop compulsive self-stimulation behavior. “Conversely, when we reduced the activity of the circuit in an addicted mouse, she stopped activating the lever!” Dr. Pascoli added.
The findings do highlight a number of questions, the researchers noted. “We found that in a subpopulation of mice that acquired optogenetic dopamine-neuron self-stimulation (oDASS), the strengthening of OFC–striatum synapses was causally linked to the perseverance of reinforcement despite punishment … An important question is how does oDASS compare to cocaine exposure … Another open question is how the plasticity that underlies compulsion is induced.”
What the researchers also don’t yet understand is why, in their mouse model, the activity of the brain circuit was different in some of the otherwise genetically identical animals than in others, and resulted in some animals resisting compulsive lever-pressing, while others became addicted to stimulating their reward systems. “Why only a fraction of mice lose control remains to be determined; the emergence of the two groups is even more surprising given the high degree of genetic homogeneity of the mouse line used here …” the team wrote.
“That’s the question we’ll try to answer in our future research”, Dr. Lüscher stated. Existing hypotheses include epigenetic differences based on life experiences that influence the gene and brain function. “Thanks to the present study, we now know which circuit causes the addiction,” commented Dr. Pascoli. “It will then be easier to find out what causes the disruption in the circuit.”