Maintaining quality production of pharmaceutical products is an essential endeavor in the pharmaceutical industry. With biopharmaceutical companies moving toward continuous processing, the FDA encourages real-time monitoring through process analytical technology, or PAT.
During PAT, the manufacturing process continually analyzes pharmaceutical samples to determine critical quality attributes (CQAs) that may fluctuate during production. Of course, global pharmaceutical companies have implemented protocols for continuous monitoring for decades; however, more sophisticated methods are emerging as advances in technology continue. In recent years, online HPLC/UHPLC has incorporated several advances including innovative software to modulate the continuous processing of proteins. With these advances, an added requirement of decreased chromatographic analysis time has emerged.
Two frequently employed chromatographic methods in PAT are ion-exchange chromatography (IEX) and size-exclusion chromatography (SEC). These methods can measure CQAs including charge heterogeneity and protein aggregation, respectively. Orthogonal analytical methods to measure these CQAs, such as capillary electrophoresis and analytical ultracentrifugation, are done during therapeutic characterization; however, for continuous processing, a more robust liquid chromatography method is preferable.
A major challenge in recombinant protein synthesis is the heterogeneity of the final product, which is dramatically different from small-molecule production. With implications for factors such as binding affinity and therapeutic efficacy, charge heterogeneity monitoring is necessary during PAT. For real-time analysis to have a direct impact on protein production, rapid charge variant analysis is critical.
A chromatographic challenge generally seen with protein analysis, but particularly with charge variant analysis, is creating an inert column surface for analysis due to the propensity of these proteins and variants to interact with traditional metallic surfaces. The most common approach to mitigate such interactions is the utility of bioinert polyether ether ketone (PEEK) column hardware. This hardware surface provides excellent recovery of protein charge variants with minimal column priming required. Unfortunately, as laboratories move toward more modern instrumentation, such as ultra-high-performance liquid chromatography (UHPLC), to speed up their analyses, PEEK hardware has back-pressure limitations and column-to-column variability.
A recent transition in column hardware to titanium instead of stainless steel offers similar biocompatibility to PEEK, but with the robustness and pressure capacity of stainless-steel hardware. With this advance, charge variant analysis can proceed at high flow rates with similar separation of acidic and basic variants from the protein target molecule. Weak cation-exchange (WCX) is the most common technique for charge variant analysis, though strong cation-exchange (SCX) is also a viable option.
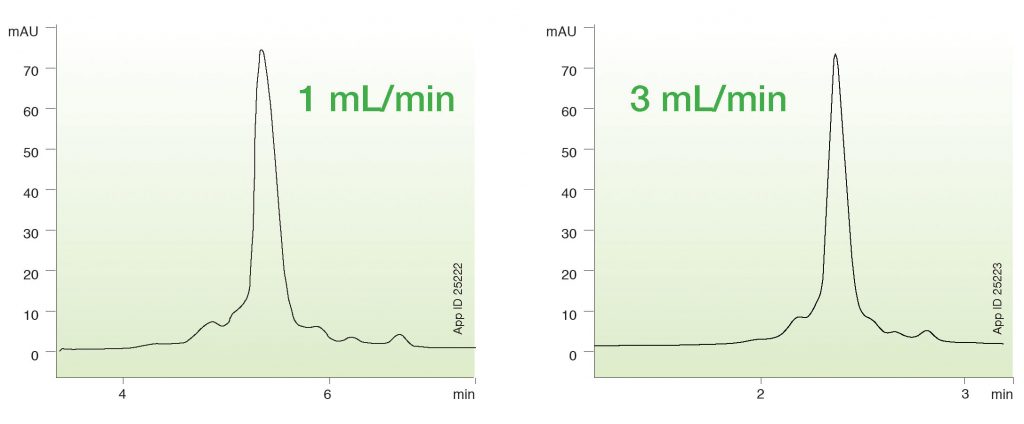
Figure 1 shows the ability of a 6 µm WCX column with titanium hardware to separate charge variants of rituximab at different flow rates. As shown, the flow rate of the analysis has minimal impact on the separation of charge variants. At 1 mL/min flow rate, the separation provides 17% acidic and 10% basic variants. Increasing the flow to 3 mL/min gives 15% acidic and 12% basic variants. With WCX, the particle surface is typically a polycarboxylate that can bind to protein variants through electrostatic charge interactions. A main reason the resolution of acidic and basic variants is not changed at a higher flow rate is because the polycarboxylate surface allows for a bind-and-elute-type mechanism, which is irrespective of flow rate. Of course, the main overall advantage is that the analysis time is reduced from 12 min to 6 min with minimal impact on the quantitative assessment of variants. These combined factors facilitate rapid, real-time monitoring for PAT.
Aggregate analysis is the other most common CQA monitored during PAT. Protein aggregation also has implications in biotherapeutic safety and efficacy, and therefore, rapid assessment of aggregate levels is necessary during production. In certain cases, the aggregate analysis is performed in parallel with preparative WCX, and therefore, reduced analysis time is critical. The advent of UHPLC systems and sub-2 µm particles has increased efficiency and reduced speed in PAT SEC methods.
Columns using sub-2 µm particles enable the use of shorter columns and higher linear velocities, and these ultimately lead to faster run times. Similar to charge variant analysis, the analytes of interest (high molecular weight aggregates and low molecular weight fragments) are prone to interaction with metal column hardware. To avoid the traditional stainless-steel columns that are known to adsorb proteins, titanium column hardware is a viable option because the high dielectric constant of titanium oxides limits protein interaction, thus making it bioinert. Moreover, bioinert UHPLC systems, which are also lined with titanium tubing, can be employed to ensure more complete biocompatibility. Again, the overall benefit here is to minimize adsorption of the protein onto the column while maintaining the robustness of a fully metallic column that can withstand high pressures at higher flow rates, ultimately leading to more accurate analysis in decreased time.
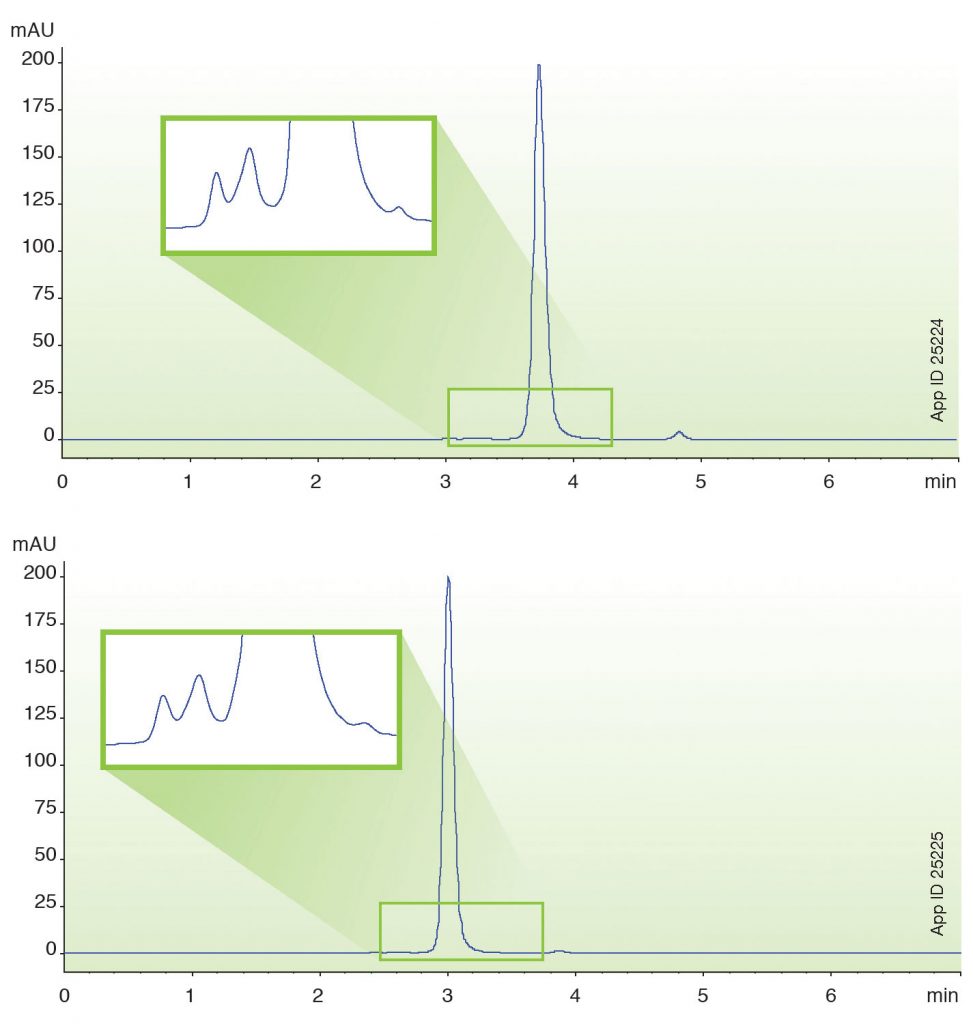
For example, Figure 2 shows the analysis of rituximab under standard UHPLC conditions (top) and under fast conditions (bottom). The percent aggregate in the two analyses remain consistent at 1% under both normal flow and fast flow. Minor fragments are also observed in each chromatogram, resulting in <1% fragment in each case.
The real-time monitoring requirement in continuous processing of biotherapeutics has placed pressure on process analytical scientists to create novel, rapid methods to assess product integrity. Advances in instrumentation and software have accelerated in recent years, making online monitoring feasible. Regarding the chromatographic monitoring of CQAs, improved separation science as well as column hardware have emerged as viable options for rapid PAT applications.
Chad Eichman, Ph.D. ([email protected]), is biopharmaceutical global market manager, Brian Rivera is global product manager, and M. Christina Malinao is biopharma scientist at Phenomenex.


