Gene and modified cell therapies (for example, chimeric antigen receptor [CAR] T-cell therapies) in early to late clinical development are showing significant promise to treat and potentially cure many diseases. A few of these next-generation drugs have already been approved and have reached the market. Despite the robust pipeline of candidates advancing through the clinic, however, there is potential for a manufacturing bottleneck—viral vector production—to delay commercialization.
Unlike most engineered protein-based biologics, which are manufactured using suspension cell culture, viral vectors require the use of adherent cells that are supported on a matrix. Two options are currently available for adherent cell growth: the use of microcarriers or multitray static plasticware.
Microcarriers are suspended in a tank and agitated, providing a homogeneous environment. The agitation can be too rough for the shear-sensitive cells required for viral vector production, however. Process scale-up requires significant effort because the hydrodynamic conditions within the reactor change. In addition, it is not possible to achieve high cell densities, and scale-up entails the use of large quantities of expensive transfection agent.
For these reasons, static plasticware has been traditionally used for adherent cell growth, transfection, and adenoviral vector production. However, this method also suffers from limitations. Precise environmental control (pH, dissolved oxygen [DO], media composition) is not possible, and extensive manual intervention is required. Furthermore, scale-up is not possible—only scale-out.
A scalable solution
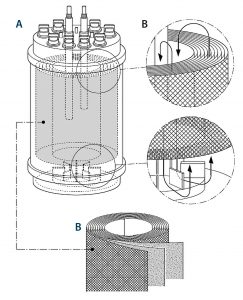
Scalable and automated single-use fixed-bed bioreactor technology from Univercells addresses these shortcomings and offers drug manufacturers the opportunity to supply the market with the required quantities in an affordable manner. A schematic of the
bioreactor is shown in Figure 1.
A tightly packed support matrix comprising spiral-wound, nonwoven polyethylene terephthalate (PET) fabric layers allows for high cell densities in a small-footprint bioreactor. A magnetic centrifugal impeller located inside the bioreactor provides two functions: good mixing to ensure even availability of nutrients throughout the fixed bed, and aeration through the creation of a “falling film” via the vessel headspace to increase the surface area available for gas exchange. The media is gently circulated across and through the cells, providing conditions similar to those present in static plasticware, but with a homogeneous environment both vertically and horizontally.
The system includes automated pH, DO, and temperature control; automated inoculum addition and manual media and cell sampling; and pumps that allow further media addition through a recirculation loop.
Scaling is achieved by increasing the height with a fixed diameter (~10-, 20-, and 30-cm height of fixed bed), then increasing the diameter in parallel (10, 20, and 30 cm). Because the linear velocity of liquid media travelling through the fixed bed remains constant across scales, the cells experience similar low-shear conditions. The automation features and ease of scale-up facilitate more rapid development and commercialization of viral vectors.
Investigation of process transfer
The ease of transferring viral vector production processes from static plasticware to the fixed-bed bioreactor was investigated by comparing the performance of processes conducted in the scale-X™ hydro bioreactor system and a standard plastic flatware cell culture flask using the same inoculation density and proportional volume of culture medium for each.
The cell-culture process in the scale-X hydro system takes place in five steps: (1) bioreactor preparation, (2) inoculation and cell attachment, (3) cell expansion, (4) viral infection, and (5) harvest (Figure 2).
HEK293 cells from a cryopreserved cell bank (Momotaro-Gene) were used. The inoculum was generated in a cell culture flask under standard conditions. The processes were monitored for cell density and virus titer (visual counts using an optical microscope) and metabolite (glucose, lactose) concentrations (off-line metabolite analyser).
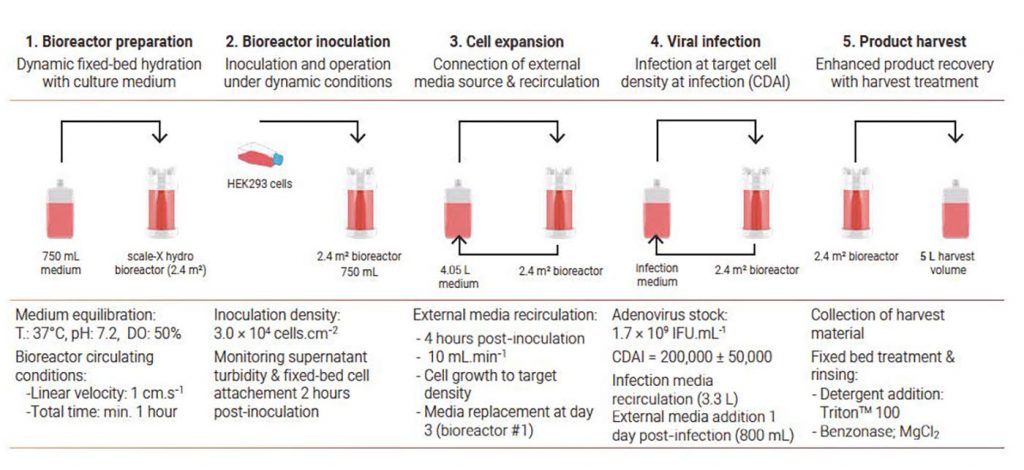
In all of the processes, an external media source was connected during cell expansion and recirculated through the bioreactor shortly after inoculation (Figure 2). In the first bioreactor (#1), the media was replaced at day 3 to allow further cell growth, but the cells were not infected. For bioreactors #2 and #3, cell expansion was performed in batch mode for 2 and 3 days, respectively, after which time the cells were infected. Although the cells naturally lyzed upon infection, product recovery was enhanced using detergent.
Successful cell growth and infection
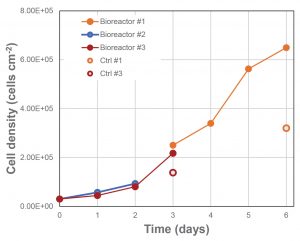
Cell growth in the fixed-bed bioreactors (#2 and #3) was higher under the same conditions than that in the standard plasticware. (The results are presented in Figure 3.) It can be seen in the figure that the fixed-bed bioreactor outperformed the standard plasticware both at day 3 (2.1 × 105 vs. 1.3 × 105 cells cm–2) and at day 6 (6.5 × 105 vs. 3.2 × 105 cells cm–2). Note that the same ratio of medium exchange was used in the plasticware and bioreactor to achieve the high cell densities observed at day 6.
All bioreactors were operated with an external medium circulation loop of 4.2 L from inoculation. At day 3, the external media circulation loop of bioreactor #1 was replaced with fresh medium, thus allowing further cell growth until day 6. Bioreactors #2 and #3 were operated in batch mode and then infected (respectively at day 2 and 3). Cell density post-infection is not shown. Control cell density for bioreactor #2 was not available.
Infection was performed at a target cell density (days 2 and 3 in bioreactors #2 and #3, respectively), and all cells were observed to be lysed 3 days post-infection. The product from each process was then recovered using the same detergent treatment. The viral titer was approximately 1 log IRU less in the bioreactor than in the control plastic flatware without performing any process development work.
Potential for further improvement with process optimization
This study demonstrated that an HEK293 process producing an adenovirus for gene therapy based on the use of static plasticware can be successfully transferred to the Univercells scale-X bioreactor system. The potential to reach very high cell densities and good viral yields within a small bioreactor volume highlights the system’s capability for high levels of production in a much-reduced footprint, and with an automated process step.
Notably, the processes in the static plasticware and the fixed-bed bioreactor gave similar results without any effort to optimize the process performed in the bioreactor. The higher cell densities obtained in the scale-X hydro bioreactor are most likely a reflection of the better environmental control (pH and DO) in the system, which promotes a stable growth environment. Combined with the promising viral yields, the higher cell densities open the door for further process development.
Future work will focus on determining the critical process parameters (CPCs) for optimization of the culture conditions in the bioreactor, a feat that is not possible with traditional static processes due to the lack of control of process parameters such as pH and DO.
The scale-X hydro system is part of a portfolio of bioreactors allowing process development and pilot scale cultures (scale-X hydro at 2.4 m2 and carbo 10–30 m2), medium-to-large-scale industrial production (scale-X nitro, 200–600 m2, typically suitable for vaccine production), and larger scale industrial production (scale-X oxo, >2000 m2, to meet the needs of gene therapy). Further experiments are also planned to demonstrate the scalability of the process to these larger scale bioreactors.
Yael Dohogne, Florence Collignon, Jean-Christophe Drugmand, Alex Chatel, and José Castillo are at Univercells. Satoshi Kumano and Hitoshi Shiomi are at Momotaro-Gene.


