Manufacturing today’s cell therapies remains expensive and complex due to the need for viruses or electric shocks to engineer patient cells. Yescarta®, for example, one of the first CAR-T therapies approved for sale, takes 3-4 weeks to reach patients and has a price tag of $373,000.00.
With CAR-T, expanding out a patient’s T-cells and transducing them with a virus is the most expensive and time-consuming step. That’s according to Armon Sharei, PhD, the 32-year-old CEO of SQZ Biotech, whose cell engineering platform was named as a top 10 world-changing technology by Scientific American in 2014.
The company was cleared for its first US clinical trials in October this year.
Sharei believes the company’s cell squeezing technology can cut manufacturing times to under 24 hours per patient with the potential to offer a wide range of cell therapies at the point of care.
“It’s not something we’re doing today but, for the future, we’re looking at systems where essentially you can take white blood cells out of patients, run them through a machine and put them straight back in,” he says.
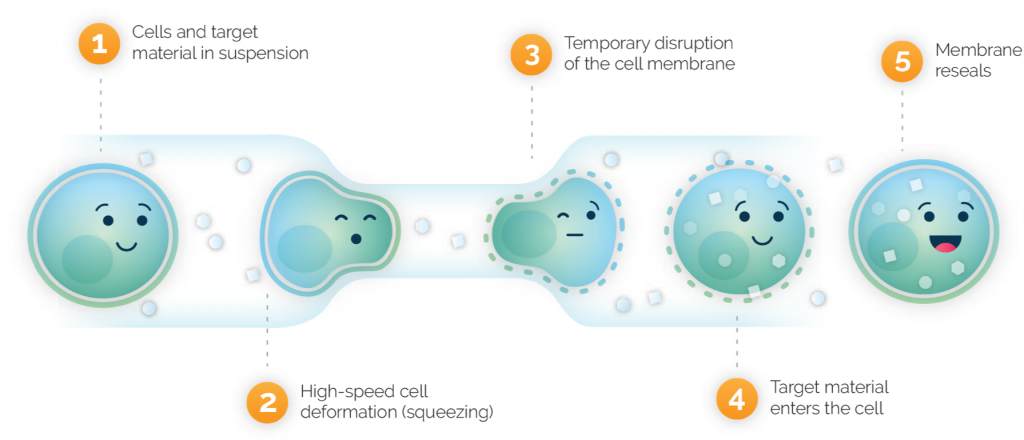
Instead, he explains, manufacturing becomes a simple matter of a wash step, mixing the cells with proteins or other materials, and running them through the chip at a rate of 10 billion per minute. And, as the limiting factor on manufacturing speed is the mechanical washing, squeezing, and formulation steps, Sharei is confident that unlike with traditional CAR-T, automation can make their processes even more time efficient.
“How much you can automate does depend on the therapeutic concept and, in traditional CAR-T, the limit is how fast cells can divide, how fast you can transduce the virus, and robots can’t make that faster,” he explains
Scaling up going into clinical trials is also easier, he argues, as the company simply needs to run more small-footprint microfluidic chips.


