June 15, 2015 (Vol. 35, No. 12)
From our June 15 Issue: HPLC’s Discriminating Power Quietly Resolves Madding Mixtures and Methodically Sequesters Compounds
When Waters introduced the first commercial high-performance liquid chromatography (HPLC) system in 1969, no one foresaw that HPLC, along with several of its significant embodiments, would come to be preeminent in life science analytics.
Walk into any pharmaceutical, environmental, or academic biology laboratory today, and you will likely encounter a 20-year-old HPLC instrument employing 10-micron columns. Almost as likely are UHPLC instruments (including Waters’ branded UPLC®), systems engineered for superficially porous particle columns, or even supercritical-fluid-based LC instruments.
The diversity of systems and columns, while welcome, raises theimportant issue of method transfer, which is of great interest in analytical biotech. Successful transfer depends on understanding chromatographic principles as well as each instrument’s operating parameters, such as temperature control, operating pressures, flow rates, and method conditions.

Successful method transfer depends on understanding key HPLC principles as well as each instument’s operating parameters. [iStock/Versevend]
Getting from There to Here
“All [method transfer] factors must be considered and accounted for when transferring methods from instrument to instrument and across vendor platforms,” says Paula Hong, Ph.D., principal scientist at Waters. Besides accounting for chromatographic principles and operating parameters, Dr. Hong has evaluated method transfer factors such as gradient delay, temperature, and sample solubility and diluent. Moreover, she has assessed method transferability through system suitability criteria such as retention time, relative retention time, and area.
In a study comparing Waters’ HPLC and UPLC instruments to competitors’ systems, Dr. Hong concluded that several well-established instrument parameters had profound effects on method transfer. “Often one talks of scaling methods from HPLC to UHPLC,” says Dr. Hong. “Here we were more interested in transfers using the same column and conditions, and understanding the impact of instrument characteristics.”
Dr. Hong looked specifically at system dwell or gradient delay volume, which was measured using an organic tracer from low to high percent organic. After manually measuring system dwell volume, Dr. Hong performed a band-spread test using an analyte that was not retained, to view dispersion effects.
Dr. Hong then ran gradient separations to study the impact of delay volume on the separation. The objective was to determine how to achieve good retention time correlation between systems with different dwell volumes under gradient changes. She found that by manipulating the gradient delay it was possible to get retention time correlation when transferring from a system of lower dwell volumes to one of larger volumes, and vice versa. This is possible by adjusting the gradient start relative to the injection without requiring any changes to the gradient timetable.
Gradient delay, or dwell volume, is the volume of liquid between the point where the gradient forms at the mixing head and the point where the mobile phase reaches the column head. Depending on volumes, the delay might be 1 mL for HPLC and 400 µL for UHPLC-class instruments.
For extra-column dispersion, Dr. Hong examined an isocratic separation with a minimally retained compound. “Extra-column dispersion is most noticeable with weakly retained compounds because their peak width is narrower,” Dr. Hong explains. “When this is the case, extra-column effects will be magnified in terms of peak broadening.”
Analysts who transfer methods without understanding instrument characteristics might not meet criteria for retention time or resolution. But adjusting the gradient delay volume allows a low-volume system, such as a UPLC or UHPLC system, to mimic a system that has a larger system volume, such as an HPLC system.
“People don’t realize that gradient delays are part of LC. They don’t always understand its impact,” comments Dr. Hong. “It’s not even in their method, it’s part of the instrument that leads to retention time shifts.”
Analyzing Excipients
Polysorbate 80 is an excipient used in biotherapeutics products to increase drug substance solubility and prevent aggregation and surface adsorption. Excipient levels in approved drug products are regulated by the FDA, and therefore require quantitation. Approved levels of polysorbate 80 are listed in the FDA Inactive Ingredient search tool online.
Several polysorbate analysis methods exist. Polysorbates, however, lack chromophores, so methods involve time-consuming derivatization and alkaline hydrolysis. Also, these methods require an additional step to remove the drug.
A group at Shimdzu Scientific Instruments led by William Hedgepeth recently reported on a simple, reliable 2D HPLC method for quantifying and characterizing the quantitation and characterization polysorbates in biotherapeutic products. A typical experiment uses 20 mg/mL of human IgG and 0.1 mg/mL of polysorbate 80 in 10 mmol/L phosphate buffer at pH 6.8. Due to its size, the antibody passes through a restricted-access C18 trap column containing 50-µm particles; sugars, salts, and amino acids also pass through because of their polarity.
After elution of the untrapped analytes, the polysorbate is then backflushed to an analytical C18 column, in this case a 5 µm core-shell column that provides good efficiency while providing low back pressures. Characterization involved a longer core-shell column with a shallower gradient to improve chromatographic resolution. Detection occurred using a Shimadzu LCMS-2020 single quadrupole mass spectrometer.
“Polysorbates are complex mixtures of polymeric compounds,” Hedgepeth says, “so many peaks were present on the total ion chromatogram.”
High-Temperature Operation
High-temperature HPLC brings with it the benefits of shorter analysis times, higher efficiency, and lower overall pressures. Hillel Brandes, Ph.D., a chemist at Sigma-Aldrich’s Supelco division, has examined these benefits for globular proteins, monoclonal antibodies, and peptides using columns packed with 3.4-micron, 400-angstrom pore size, C4 alkyl-bonded, superficially porous particles.
The rates of chemical reactions rise rapidly with higher temperatures. Could this affect the stability of proteins during analysis?
Dr. Brandes admits that such concerns are legitimate: “The needs, timeframe, and nature of the chromatographic separation may dictate the application of conditions for which the protein is no longer in a native conformation, nor may it be even desirable. Recovery of the native protein structure or activity is usually not an objective with analytical characterization.” For example, reversed-phase separation of proteins or peptides is conducted at low pH to optimize the separation, and for compatibility with the separation media, irrespective of the effects on analytes.
Dr. Brandes injected protein and peptide standards onto short 2.1 mm ID columns running an aqueous acetonitrile/trifluoroacetic acid gradients at temperatures of up to 90°C for 15,000–60,000 column volumes. He found that retention time stability persisted longer for monoclonal antibodies and globular proteins than for peptides; efficiency and peak shape changed minimally or not at all.
Retention time differences at 90°C vs. 35°C varied by as much as 25% or more, but this effect was not, according to Dr. Brandes, a function of molecule class. Declines were modest for proteins compared with peptides, which Dr. Brandes attributes to differences in gradient steepness parameters.
“At 35°C, we did not observe a decline in retention time, nor were peak shape and efficiency impacted for any of the biomolecules studied,” Dr. Brandes notes. These results illustrate the importance of optimizing temperature when performing biomolecule separations.
“Retention times were more reproducible over time at higher temperatures for the proteins, perhaps because, in comparison to peptides, retention of proteins in reversed-phase chromatography relies less on partition and more on adsorption,” Dr. Brandes explains.
In other words, bonded phase hydrolysis that may occur at low pH and accelerate at high temperature affects partitioning of peptide analytes between the stationary phase and the mobile phase more than proteins that are more strongly partitioned into the stationary phase to begin with.
New HIC Chemistries
Size-exclusion chromatography (SEC), ion-exchange chromatography (IEX), and reversed-phase chromatography (RPC) are the principal HPLC modes used for characterization of unmodified monoclonal antibodies (mAbs). Hydrophobic interaction chromatography (HIC) has also been used for analysis of mAbs and related substances including succinimides, antibody fragments, oxidants, C-terminal lysine variants, and drug conjugates.
“HIC is not as widely used as the major three chromatography modes because not many chromatographers are familiar with it,” says Julia Baek, Ph.D., associate research scientist at Thermo Fisher Scientific. “Although not as well known as other separation modes, HIC provides orthogonal selectivity to SEC and IEC; therefore, some mAb variants can be better separated by HIC compared to SEC or IEC, and vice versa.”
Thermo Fisher recently introduced a new family of HIC columns for mAb analysis, namely MAbPac HIC. These columns exhibit the desired and complementary selectivity for mAb separations, excellent recovery, high efficiency, and chemical stability. The company’s MAbPac HIC columns are made of wide-pore silica particles or polymer nonporous resins specifically designed for mAb and mAb variant analysis.
“We have separated quite a few challenging samples that our customers could not separate using other modes or even other marketed HIC columns,” asserts Dr. Baek. “Two examples are a fusion protein and a bispecific mAb.”
By themselves, mAbs exhibit significant heterogeneity due to post-translational modifications and biochemical degradation. Most mAbs on the market are of the IgG1 type, but variations are now under investigation. And through advanced protein engineering technologies, researchers are designing mAbs for higher potency and stability, as well as other desirable properties.
Such modifications often add heterogeneity to the molecule. For example, conjugating a drug or a polyethylene glycol (PEG) molecule on lysine residues of a mAb potentially yields 28 or 29 variants based on the number of drug or PEG molecules attached and the number of positional isomers (assuming there are eight or nine lysine residues on the surface of the mAb). Molecular variability has therefore given rise to the search for highly resolving HPLC modes and for columns with novel chemical functionalities.
Recovery is normally not an issue with analytical HPLC, but Dr. Baek notes that in some instances investigators will need to collect a fraction for downstream analysis. “As opposed to RPC, which also separates mAbs based on hydrophobicity under denatured conditions, HIC runs under nondenatured conditions and thus preserves the native structure of the protein.”
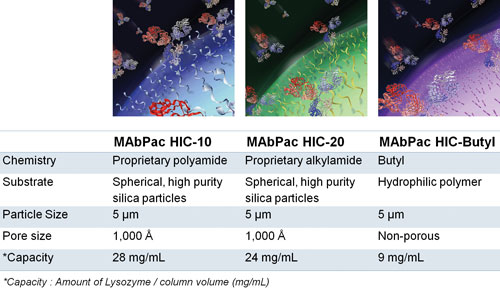
The MAbPac HIC columns constitute Thermo Fisher Scientific’s aptly named family of hydrophobic interaction chromatography columns for monoclonal antibody (mAb) analysis. The three MAbPac HIC columns shown here provide complementary selectivity and the ability to separate a range of mAbs, mAb fragments, antibody-drug aggregates, and related biologics. These columns, the company adds, combine efficiency, biocompatibility, and ruggedness.
Glycan Analysis
The significance of glycosylation to drug activity and half-life requires bioprocessors to understand and control it, to the degree possible, during glycoprotein drug development and manufacturing. N-linked glycan structural profiles must be characterized fully during development of new biopharmaceuticals and throughout the development of biosimilars and follow-on products.
William Long, Ph.D., of Agilent Technologies has reported on novel glycan sample preparation methods, amide-based hydrophilic interaction liquid chromatograph (HILIC), and fluorescence-mass spectrometric detection for analyzing IgG N-linked glycans. The new sub-2 µm fully porous and 2.7 µm superficially porous HILIC columns enable high resolution and significantly lower glycan elution times compared with currently available LC methods.
Agilent’s new AdvanceBio Glycan Mapping columns are based on a novel amide chemistry bonded to silica particles for UHPLC. The columns excel at resolving complex mixtures of N-glycans with subtle structural variations, according to the company.
“Structural profile” refers to the relative quantity of each unique N-glycan structure compared to all the other N-glycan structures in the sample, Dr. Long explains: “The goal is to assign these structures with a high degree of specificity and confidence. This is the most important type of glycosylation analysis performed in the biopharmaceutical industry. But structural profile is not the whole story, since we do not know how those structures are distributed across the population of proteins with multiple glycosylation sites.”
For example, the profile does not indicate whether some copies of the protein contain zero N-glycans. Alternative methods such as LC-MS peptide mapping and intact mass analysis can answer those questions, but they typically give ambiguous glycan structural information and lower quantitative accuracy compared to the structural profile obtained by HILIC UHPLC of cleaved glycans. N-glycans are typically cleaved from protein using PNGase F, a standard approach.
“Glycosylation is a critical quality attribute that should be investigated at multiple manufacturing stages during process development, in addition to monitoring in the final product,” Dr. Long continues. “A thorough understanding of how the production parameters affect glycosylation will become crucial if the glycosylation profile of the product changes in future product batch.”



