Nature has spent millennia honing the virus into a ruthlessly efficient delivery vehicle for nucleic acids. Viruses have even been harnessed for our own delivery purposes. But some applications have had only mixed success. For example, commercial applications of genetic engineering, which require high scalability, low cost, and impeccable safety, remain a challenge.
Although they can easily enter the body and inject their payload into cells, viruses may stimulate a dangerous immune reaction and cause long-term medical complications. In addition, viruses can be expensive and time consuming to cultivate.
Safer and more practical alternatives to viruses are being sought by innovative companies. For example, these companies are developing nonviral gene delivery systems that incorporate nanoparticle formulations, ultrasound, and electric fields. These systems can slip bits of genetic material into cells efficiently and cost-effectively in a range of applications.
Needle-free vaccines that need no refrigeration
All eyes are on vaccines right now, as companies race to bring a safe and effective SARS-CoV-2 vaccine to market. Although mRNA vaccines have certain advantages over viral particles, such as ease of manufacturing and lack of infectious potential, they need to be kept refrigerated or frozen, making distribution a challenge.

Vaxess Technologies is developing a COVID-19 vaccine that can be delivered with the company’s MIMIX™ system, a shelf-stable, single-dose skin patch that could vastly improve vaccine administration. Although other formulations require multiple injections, the Vaxess vaccine in combination with the MIMIX system needs to be applied just once, and the skin patch is simple enough to be administered at home.
MIMIX uses the silk protein fibroin to stabilize the antigenic molecule, allowing the vaccine to be stored and transported at room temperature. The fibroin protein can be “tuned” to control the rate that the vaccine diffuses into the body.
“I’ve never seen a material that can do what this material can do,” says Michael Schrader, co-founder and CEO of Vaxess. Schrader, a mechanical engineer with a background in plastics, continues, “We’re able to make constructs precisely engineered for whatever application we need.”
The MIMIX patch is applied for five minutes, embedding dozens of silk-based tips into the skin. Upon removal, the tips remain in the skin, where they gradually release the antigen formulation into the body over a period of weeks. Antigen formulations under development by Vaxess include a SARS-CoV-2 spike protein and a lipid-nanoparticle-encased mRNA that encodes a SARS-CoV-2 spike protein.
When a virus infects the body, a robust immune response develops in stages, over a period of two to three weeks. As the virus replicates, antigens are shuttled to the lymph nodes, where the appropriate T cells and B cells are activated. In contrast, the body clears most killed-virus or protein vaccines from the body within a day or two.
“The concept of our system,” Schrader explains, “is to use sustained release to fool the immune system into thinking there’s an actual infection, and to get that high quality and high quantity of antibodies and T cells.” The system presents an opportunity to counter COVID-19 with a single-dose vaccine. One MIMIX patch can provide a level of protection that typically requires two separate injections. The sustained-release system also allows delivery of more material with less reactogenicity than a single huge dose. “That has been one of the biggest limits of dosage,” Schrader maintains. “By spacing out cellular uptake, with a patch instead of a bolus, we can potentially reduce the amount of cell death. It’s a beautiful compound effect that can lead to a much more potent immune response as well as an improved patient experience.”
Instant COVID testing, courtesy of CRISPR
While Vaxess is getting SARS-CoV-2 RNA into the cell, Sherlock Biosciences is getting it out.
Testing for COVID-19 is cumbersome and unpleasant, and it can take days to get the results back. Sherlock seeks to vastly simplify and speed up this testing.

SHERLOCK (Specific High-sensitivity Enzymatic Reporter unLOCKing) is the technology behind a rapid, point-of-care test that combines a CRISPR enzyme, Cas13, with a gRNA specific to SARS-CoV-2 RNA. First, the sample RNA is amplified using an isothermal amplification. Then, the guide RNA binds the viral target sequence, activating the Cas13 enzyme. Once activated, Cas13 cuts the labeled nucleotide sequences added to the reaction, producing a positive signal. “We use CRISPR to generate the signal by cleaving labeled probes,” says Rahul Dhanda, co-founder and CEO of Sherlock. “And those probes are what we detect.”
In July, Sherlock announced a partnership with Binx Health that will combine Sherlock’s CRISPR technology with the Binx io platform, a desktop-sized device that incorporates single-use multiplex cartridges and is capable of testing up to 24 targets.
The test will be available in a variety of CLIA-waived settings, including pharmacies, outpatient clinics, and assisted living facilities. “We had identified that one of the big areas of need is really around decentralized testing,” Dhanda points out. “It’s for those people who can’t get the hospital, or those who are unwilling to.”
Sherlock also envisions a simple, instrument-free test that can be done at home, like a pregnancy test. This test, which uses Sherlock’s INSPECTR (Internal Splint-Pairing Expression Cassette Translation Reaction) technology, incorporates synthetic biology sensors that are activated only in the presence of SARS-CoV-2 RNA.
The test relies on the cell’s transcription and translation machinery, dried onto a paper strip. A gene encoding a signal molecule is synthesized, broken into pieces, and imbued onto the test strip. These pieces include a few base pairs that match up to the target RNA that is to be detected.
“If the virus is present, it will hybridize to the virus, in order, and reassemble itself,” explains Dhanda. Then the cell-free system in the test strip will transcribe and translate that reassembled gene, producing the signal molecule. The nature of the test ensures extremely high specificity, and it can be programmed to seek out whatever sequence is needed.
“It’s an extremely elegant system,” Dhanda asserts. “It can be used at room temperature, without an instrument, with mass-produced lateral flow equipment. When it became clear this was not a virus that is going to burn out at the end of spring, we recognized that what we really need to do is come up with a home test.”
To infiltrate cancer cells, think positive
Even in a pandemic, cancer does not take a break. Lung cancer remains the deadliest cancer in the United States, with few effective treatments available. Some 80% of non-small cell lung cancers have nonfunctional copies of the tumor suppressor gene TUSC2.
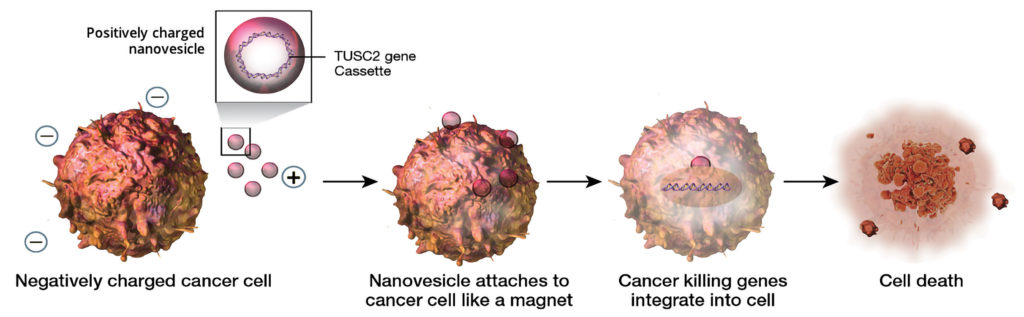
Genprex, a clinical-stage gene therapy company, uses a nanoparticle delivery
system called Oncoprex to get TUSC2 into the cells and trigger apoptosis. To deliver plasmids bearing TUSC2, Genprex envelops them inside lipid nanoparticles designed to be positively charged. These nanoparticles consist of a surfactant (DOTAP) and cholesterol. The combination of DOTAP, which is strongly cationic, with cholesterol, which is charge neutral, creates a very stable, long-lasting particle that doesn’t form aggregates and can fuse with the cell membrane, depositing the nucleic acid into the cell.
The positive charge is the key to the particles’ specificity. Cancer cells burn copious amounts of sugar to fuel their endless rounds of division. This high metabolic activity generates lactate ions that create a negative charge on the cell surface. The positively charged nanoparticles preferentially home in on the cancer cells.
“Tumor biopsies have shown that the nonviral nanoparticles are taken up by cancer cells at 10 to 25 times the rate they’re taken up by normal cells,” says Rodney Varner, founder and CEO, Genprex. “We’ve treated over 50 patients with this system, and it’s shown a favorable safety profile.”
“Oncoprex on its own does two things,” explains Michael Redman, executive vice president and COO of Genprex. “It introduces the TUSC2 suppressor gene into the cell by endocytosis, and on top of that, it has an immunostimulatory effect.”
Genprex has several therapies in the pipeline that use Oncoprex. The TUSC2 therapy is called GPX-001. It is, according to preclinical data, synergistic with both targeted therapies and immunotherapies. Genprex recently received fast-track designation from the FDA for GPX-001 combined with Tagrisso (osimertinib), AstraZeneca’s targeted therapy against non-small cell lung cancer driven by epidermal growth factor receptor (EGFR) mutations. A Phase I/II trial of GPX-001 with Tagrisso is expected to begin early 2021. The company is also initiating a Phase I/II trial of GPX-001 in combination with Merck’s immunotherapy drug, Keytruda (pembrolizumab).
“Our drug is likely applicable to a wide range of other cancers,” Varner asserts. “There’s preclinical data showing that it might be effective in a whole series, including triple-negative breast cancer.”
The power of sound
Like an opera diva shattering a wine glass with her pure high C, Suono Bio uses the power of soundwaves to breach the cell membrane. For a variety of cargos, including small-molecule drugs, biologics, and nucleic acids, Suono has used ultrasound to successfully deliver the desired molecule into the cell.
“Most people are familiar with ultrasound for imaging applications,” says Carl Schoellhammer, PhD, who helped found Suono and is currently its COO. “If you use ultrasound at a lower frequency than what’s used for imaging, you can achieve delivery.” Suono’s focus so far has been in the gastrointestinal tract, delivering medications to treat inflammatory bowel diseases.
First, a fluid containing the active molecule is introduced into the colon. The ultrasound generates tiny bubbles in the fluid, which then implode, producing a jet forceful enough to carry the drug across the cell membrane. The whole process takes under a minute—a huge improvement over current inflammatory bowel disease treatments, which require the patient to tolerate an enema for hours while waiting for the medication to be absorbed.
In addition to delivering anti-inflammatory small-molecule drugs, Suono scientists have used the ultrasound technique to deliver small interfering RNA (siRNA) that blocks production of the inflammatory molecule tumor necrosis factor (TNF)-α. “This is a completely naked RNA—no lipid, nothing,” stresses Schoellhammer. When the scientists delivered the RNA to mice with colitis, levels of TNF-α dropped significantly, suppressing inflammation. Results were achieved with very low dosages, less than 100 ng of RNA, indicating the therapy could scale up for use in humans.
The method’s beauty is that it is “formulation agnostic,” Schoellhammer says. The method has been used to deliver small-molecule drugs, biologics, siRNAs (to turn off genes), and mRNAs (to produce proteins). “If we have a new drug target,” he declares, “we can immediately take that siRNA and start delivering it in animals, as opposed to having to do a tremendous amount of formulation work at the beginning just to prepare the siRNA to be delivered.” Also, the hyperfocused delivery method reduces the likelihood of off-target effects. Without the protection of a chemical formulation, nucleic acids generally won’t survive long enough to get taken up by cells outside the target area.
“You’ll most likely have almost no effect anywhere else in the body because the delivery is only where we place the technology,” says Schoellhammer. For now, the company is focusing on delivery methods to the colon. One method uses a controller-tethered system that passes through a colonoscope. Eventually, an ingestible pill may be deployed that contains all the electronics and ultrasound components.
Just a little buzz
Cell therapies can demand a time-consuming process of inserting DNA into T cells, growing them in quantity, then reinfusing them into the patient. For cancer patients, each day spent waiting for the therapy allows the disease to gain ground.
Accelerating the process may be possible with a new method that continuosly passes T cells through a pulsed, low-energy electric field. Electroporation is already used by cell biologists to deliver DNA or RNA into cells. The new method, which is being developed by Kytopen, uses less electrical energy to open pores in cell membranes.
“Cells do not like to be shocked,” says Paulo A. Garcia, PhD, co-founder and CEO of Kytopen. “By using one-fifth of the electrical energy of other commercial systems, we can deliver to the nucleus, but maintain cell viability and cell function.”
The system, called Flowfect™, keeps the cells continuously flowing, which reduces the amount of time the cells are exposed to the electric field and immediately delivers the cells to the recovery media. “CAR T-cell therapy requires on the order of 100 million CAR T cells,” Garcia notes. “We can process cells at 1 billion cells per minute.”
According to Cullen R. Buie, PhD, an associate professor of mechanical engineering at MIT who co-invented Flowfect technology and co-founded Kytopen, “Many systems have limitations in one way or another.” Often, systems pose tradeoffs involving cell health, throughput, and delivery to the nucleus. “Our technology,” he declares, “offers what we believe is the best mix of all those things.”
Kytopen has transfected an array of cell types, including both activated and naive T cells, natural killer cells, monocytes, macrophages, and induced pluripotent stem cells. The stem cells retain the ability to differentiate into different lineages after transfection.
“The diversity of cell types and payload combinations, and thus the diversity of applications that we will be able to go after, is pretty broad,” Buie states. “For all of these cell types,” adds Garcia, “we’re always using the same buffer and the same devices, and what is changing is the programs we run.” Once the company establishes the parameters that ensure high viability and high expression for a particular cell type, the process can easily be scaled up to process a large sample volume.


