A drug developed by Salk Institute researchers acts like a master reset switch in the intestines and can prevent and reverse intestinal inflammation in mouse models of inflammatory bowel disease (IBD). The compound, called FexD, had previously been found to lower cholesterol, burn fat, and ward off colorectal cancer in mice. Results from the team’s newly reported study, reported in PNAS, now suggest that by targeting FXR, a protein that signals in response to bile acid production, FexD could be developed as a treatment option for IBD.
“In people with IBD, our strategy could potentially be very effective at preventing flare-ups and as a long-term maintenance drug,” said first author Ting Fu, PhD, previously a postdoctoral fellow at Salk and now an assistant professor at the University of Wisconsin-Madison. “The Salk-developed drug FexD provides a new way to restore balance to the digestive system and treat inflammatory diseases that are currently very difficult to manage,” added Salk Professor Ronald Evans, PhD, director of Salk’s Gene Expression Laboratory and March of Dimes Chair in Molecular and Developmental Biology.
Senior author Evans, Fu, and colleagues reported on their study in a paper titled “FXR mediates ILC-intrinsic responses to intestinal inflammation,” in which they concluded, “With synergistic effects in both intestinal epithelial and immune cells, these findings implicate FXR agonism as an integrative therapeutic strategy for chronic intestinal inflammation.”
Inflammatory bowel disease, which includes both Crohn’s disease (CD) and ulcerative colitis (UC), is characterized by an excess of immune cells and inflammatory signaling molecules known as cytokines in the gut. “Inflammatory bowel disease (IBD) is a chronic intestinal disorder that affects 4 million individuals worldwide,” the authors noted. “While the underlying cause(s) is not fully understood, defective epithelial barrier function and aberrant intestinal immune responses contribute to the inflammatory responses that drive the chronic disease.” Existing treatments, which mostly work by either suppressing the entire immune system or by targeting individual cytokines, are only effective for some patients and carry a host of side effects.
For more than two decades, Evans’ lab has studied Farnesoid X receptor (FXR), a master regulator protein that senses bile acids (BAs) delivered to the digestive system to help digest food and absorb nutrients. When FXR detects a shift in bile acids at the beginning of a meal, it prepares the body for an influx of food by flipping on and off dozens of cellular programs related to digestion, blood sugar, and fat metabolism. Bile acids, the team continued, are cholesterol-based metabolites that regulate mucosal homeostasis and inflammation. “In addition to solubilizing fats, BAs function as key signaling molecules involved in the regulation of metabolic processes … Notably, bile acid levels are increased in IBD, in concert with decreased FXR signaling in the ileum of Crohn’s disease patients.”
In 2015, Evans and his colleagues developed a compound called fexaramine, which activates FXR in the gut. The drug, they initially showed, can stop weight gain and control blood sugar in mice. In 2019, the researchers demonstrated that FexD—an updated version of fexaramine—also prevented cancer-associated changes to stem cells in the gut.
Their work indicated that FXR plays a role in regulating inflammation. “Every time you eat, you’re causing small amounts of inflammation in your gut as your intestinal cells encounter new molecules,” said Senior Staff Scientist Michael Downes, PhD, co-corresponding author of the newly released paper in PNAS. “FXR makes sure inflammation stays under control during normal feeding.” The authors noted, “The pleiotropic actions of the Farnesoid X Receptor (FXR) are required for gut health, and reciprocally, reduced intestinal FXR signaling is seen in inflammatory bowel diseases.”
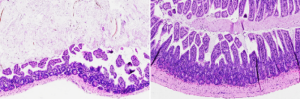
Through their newly reported work, Evans’ group discovered that activating FXR can ease symptoms in inflammation-driven diseases. When the researchers gave mice with IBD a daily dose of oral FexD, either before or after the onset of intestinal inflammation, the drug prevented or treated the inflammation. “Here, we show in an aggressive DSS-induced inflammation model that attenuation of the innate immune cell response contributes to the protective effects of FXR activation,” they explained.
By activating FXR, FexD reduced the infiltration of a class of highly inflammatory immune cells called innate lymphoid cells (ILCs). In turn, levels of cytokines already implicated in IBD decreased to levels normally seen in healthy mice. “Prophylactic activation of FXR restored homeostatic levels of pro-inflammatory cytokines, most notably IL17,” they commented. “Establishing FXR as an intrinsic regulator of ILCs provides a functional connection between diet and innate immunity.” Senior Research Scientist Annette Atkins, PhD co-author of the study, added, “When we activate FXR, we restore appropriate signaling pathways in the gut, bringing things back to a homeostatic level.”
Since FXR acts more like a reset button than an off switch for the immune system, cytokines are not completely blocked by FexD. This means that the immune system continues functioning in a normal way after a dose of FexD. The compound still must be optimized for use in humans and tested in clinical trials, but the researchers say their findings provide important information about the complex links between gut health and inflammation and could eventually lead to an IBD therapeutic.
“This study elucidates a previously unknown role for FXR in regulating innate lymphoid cells (ILCs), wherein activation of FXR abrogates ILC3-dependent intestinal inflammation,” the authors commented. The findings, they added, “support a key role for FXR signalling in the differentiation and functional maturation of intestinal ILCs in response to an inflammatory challenge and, combined with the secondary effects on T cell responses, point to the potential of FXR as a therapeutic target in intestinal inflammatory diseases such as IBD.”