Researchers at the Murdoch Children’s Research Institute say they have successfully engineered human immune cells to model an infection common among immunocompromised people.
The findings are published in the journal Stem Cell Reports in an article titled, “Human pluripotent stem cell-derived macrophages host Mycobacterium abscessus infection.”
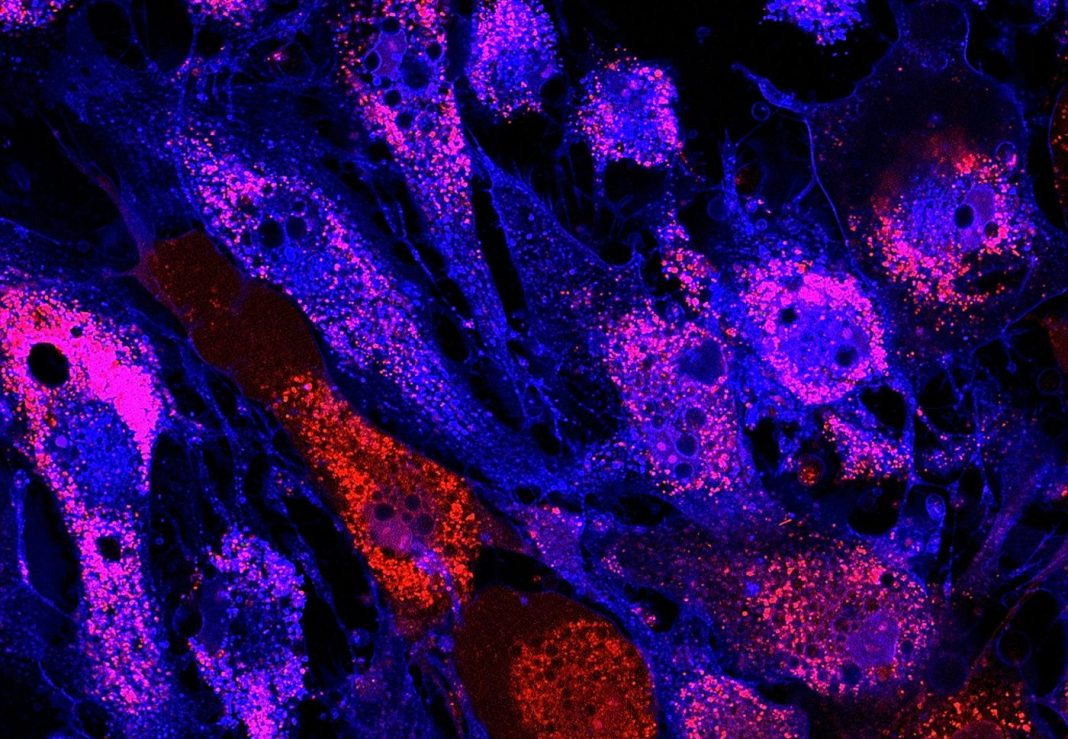
“Human macrophages are a natural host of many mycobacterium species, including Mycobacterium abscessus (M. abscessus), an emerging pathogen affecting immunocompromised and cystic fibrosis patients with few available treatments,” wrote the researchers. “The search for an effective treatment is hindered by the lack of a tractable in vitro intracellular infection model. Here, we established a reliable model for M. abscessus infection using human pluripotent stem cell-derived macrophages (hPSC-macrophages).”
Murdoch Children’s researcher Shicheng Jacky Sun, PhD, said the immune cell type the team created in the lab, known as a macrophage, played an important role in infection, inflammation, and regeneration. But this function was also a natural host for germs.
“Using our stem cell-made immune cells, we successfully infected them with a germ called mycobacteria. We could see where these mycobacteria live inside human immune cells and the immune reactions they triggered,” he said.
“We were also able to use our stem cell model to rapidly test and screen different types of antibiotics against mycobacterium.”
Murdoch Children’s said the search for effective treatments had been hampered until now by the lack of infection models to test new drugs.
“Mycobacteria opportunistically infect people with lung diseases, such as cystic fibrosis, and also causes skin and soft tissue infections in those with immunodeficiencies,” said researcher Sohinee Sarkar, PhD.
“Current treatments take months and involve giving cocktails of different antibiotics with wide-ranging toxicities. Treatments often fail as the infection is highly resistant to antibiotics, leaving infected people with few other options. Patients with mycobacteria are also excluded from receiving life-saving lung transplants.
“Improved treatments could mean less frequent hospital visits, shorter stays, and minimal exposure to toxic antibiotics, which is particularly important for children with cystic fibrosis,” she said.
“Some bacteria have evolved to escape our immune system by hiding themselves within host cells, making it difficult to treat these infections with traditional antibiotics,” she said. Our stem cell-based infection model can be readily scaled up to screen a large number of drugs against such bacteria to identify new treatments.”
“Our study describes the first to our knowledge hPSC-based model for M. abscessus infection, representing a novel and accessible system for studying pathogen-host interaction and drug discovery,” concluded the researchers.