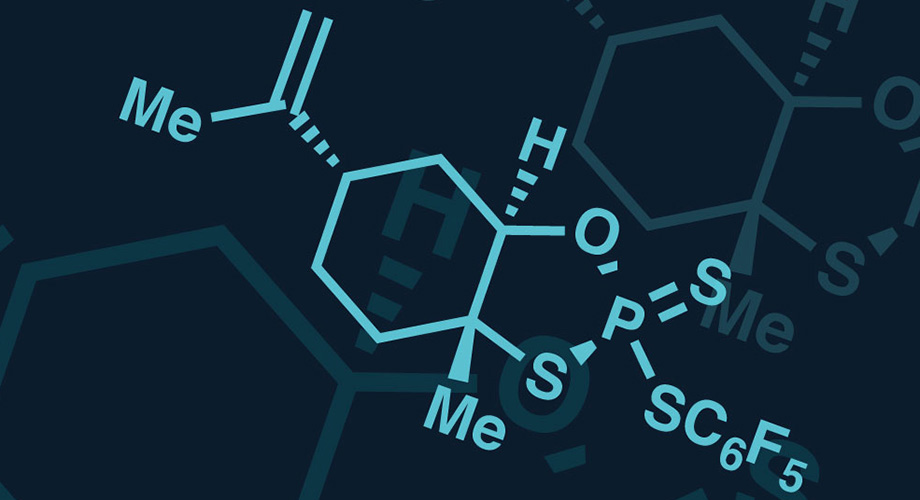
Scientists at Scripps Research and Bristol-Myers Squibb have created a novel tool for precisely controlling the stereochemistry of thiophosphates, found in some promising new drugs that target genetic molecules and other disease targets.
Called phosphorus-sulfur incorporation (PSI), the technology acts like an atomic glue gun, binding nucleosides into oligomers with specific, preprogrammed spatial configurations at the thiophosphate linkage, according to the team. These linkages are analogues of nature's method of connecting nucleosides and offer multiple advantages to drug development, but add the complexity of stereochemistry at the phosphorous atom, note the researchers, who add that PSI provides an inexpensive and simple method to enable the development of single isomers of these compounds, which can have hundreds of thousands of stereoisomers.
The study (“Unlocking P(V): Reagents for chiral phosphorothioate synthesis”) appears in Science.
“Phosphorothioate nucleotides have emerged as powerful pharmacological substitutes of their native phosphodiester analogs with important translational applications in antisense oligonucleotide (ASO) therapeutics and cyclic dinucleotide (CDN) synthesis. Stereocontrolled installation of this chiral motif has long been hampered by the systemic use of P(III)-based reagent systems as the sole practical means of oligonucleotide assembly. A fundamentally different approach is described herein: the invention of a P(V)-based reagent platform for programmable, traceless, diastereoselective phosphorus–sulfur incorporation. The power of this reagent system is demonstrated through the robust and stereocontrolled synthesis of various nucleotidic architectures, including ASOs and CDNs, via an efficient, inexpensive, and operationally simple protocol,” write the investigators.
“Thiophosphate based nucleotide compounds represent remarkable therapeutic potential, but our understanding of these systems has been hindered an inability to easily control the stereochemistry of the thiophosphate during drug synthesis,” says Phil Baran, Ph.D., a Scripps Research professor and senior scientist on the study. “PSI provides a robust and stereo-controlled method of synthesizing oligonucleotide drugs, allowing us to create, analyze, and manufacture stereoisomers of a drug candidate in ways that were previously only possible with expensive and inefficient methods.”
Martin Eastgate, Ph.D., co-senior author on the Science paper and lead scientist on the Bristol-Myers Squibb team and group director and head of chemical research in Bristol-Myers Squibb's Chemical and Synthetic Development organization, says that by providing a simple and generalized method for controlling the stereochemistry of the phosphorus-centered bonds, PSI overcomes a significant hurdle to discovering the next generation of innovative medicines.
“The invention of these stereoselective, simple, scalable, and stable reagents provides a solution to this complex problem,” says Dr. Eastgate. “We hope the invention of the PSI reagent class will prove to be an enabling technology to the scientific community.”
To build the long chain of nucleotides present in oligonucleotides, the current manufacturing technique relies on the unnatural, but highly reactive, phosphorous(III) oxidation state, he explains. One of the major limitations of applying standard P(III) chemistry to thiophosphate synthesis is a lack of control over the 3D shape of the new phosphorous based stereocenter, says Justine deGruyter, a Scripps Research graduate student and one of the first authors on the Science paper.
“Using P(III) chemistry to produce even a modest amount of the compound as a single stereoisomer is challenging, making it difficult to fully assess the impact of molecular shape on biological function,” continues deGruyter.
To overcome these limitations, the Bristol-Myers Squibb and Scripps researchers explored using a different form of phosphorus, P(V). While P(V) is generally less reactive than P(III), which can make it more challenging to use in building molecules in the laboratory, the scientists suspected its superior stability could translate into far better control over the three-dimensional molecular shape during synthesis.
Over the course of two years, the Scripps and Bristol-Myers Squibb teams collaborated to develop an effective method of using P(V) to produce desired stereoisomers of molecules. They focused on finding a way to bind together chains of nucleosides with a traceless reagent that wouldn't leave behind unwanted atoms. The result of this was the reagent PSI.
The researchers have used PSI to generate pure stereoisomers of cyclic dinucleotides (CDNs), the basis of CDN drug candidates that have shown promise as a new type of cancer immunotherapy. CDN drugs target the protein STING (STimulator of INterferon Genes) to activate the body's immune system against cancers.
“CDNs show incredible promise for activating the immune system against cancers, but until now there was no simple way to control their stereochemistry,” says Kyle Knouse, a graduate student in Dr. Baran's lab and first author on the Science paper. “The ability to efficiently and inexpensively create pure stereoisomers will provide a powerful tool to advance CDN research.”
In the case of CDNs, and ASO drugs, the ability to prepare a single stereoisomer will enable scientists to explore what shapes of the drugs are most therapeutically effective and generate those stereoisomers for clinical use. Another advantage of PSI is that it is traceless, thus avoiding the time and cost of having to remove it from the drug product during manufacturing, notes Knouse.