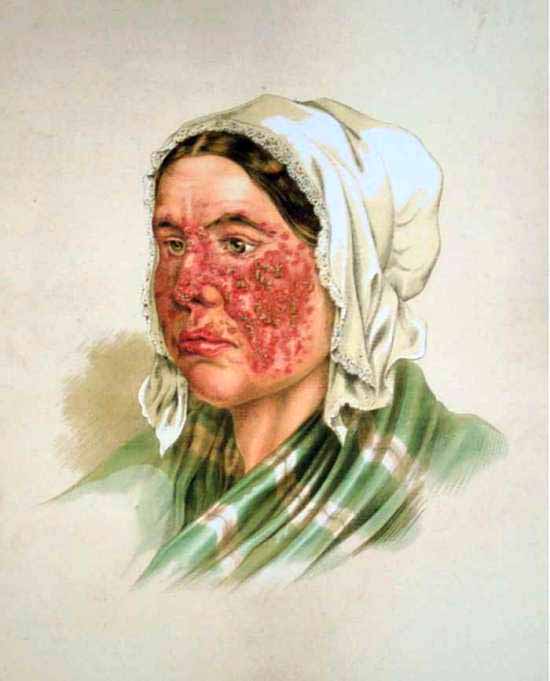
Historical drawing depicting the common “butterfly rash” most associated with lupus. [WikiCommons]
Autoimmune disorders continue to be enigmatic for the scientific community, as researchers are still unsure as to how these complex disorders are initiated. Moreover, these immunological maladies preferentially affect women with, for instance, nine out of ten systemic lupus erythematosus (SLE) cases being female. As most autoimmune disorders can take an average of up to 5 years to diagnose, combined with the fact that there are no cures for the vast majority of diseases in this category, researchers are constantly trying to find better diagnostic measures and ultimately the root cause.
Now an international team of investigators at Wake Forest Baptist Medical Center, Oklahoma Medical Research Foundation, King's College of London, and Genentech have just published data from a new study that analyzed genetic data from 27,574 individuals of European, African American, and Hispanic ancestry, identifying a large number of new genetic markers that predispose individuals to SLE. Findings from the new study—published recently in Nature Communications in an article entitled “Transancestral Mapping and Genetic Load in Systemic Lupus Erythematosus”—are important as SLE is also two to three times more common among African-American and Hispanic women.
“This study is the largest multiethnic lupus genetics study to date and allowed us to identify many new genetic markers, some of which are specific to individual ethnic groups and others that are shared across ethnicities,” noted lead study investigator Carl Langefeld, Ph.D., professor of biostatistical sciences at Wake Forest School of Medicine. “With this information, we can begin to better understand the differences in the rates and severity of disease across ethnic groups.
Autoimmune diseases strike one in 15 Americans, are among the top 10 causes of death in women, and cost an estimated $100 billion a year in medical care. SLE is the most common type of lupus and is a prototypical autoimmune disease. SLE can affect many parts of the body, including joints, skin, kidney, heart, lungs, blood vessels, and brain. Current treatments are aimed at reducing the inflammatory immune response and/or suppressing much of immune system functions. The research team is optimistic that their findings will foster new research endeavors that could lead to more specific therapeutic compounds.
“We observed that many of the genetic markers associated with lupus are shared across numerous autoimmune diseases, and those that are not shared may allow us to understand why a person develops lupus instead of another autoimmune disease,” Dr. Langefeld remarked. “These results will help us identify the biological pathways that pharmaceutical companies may target, and ultimately, develop personalized medicine for the treatment of lupus.”
[www.osmosis.org].
Using an Immunochip, a genotyping technology designed specifically for autoimmune diseases, the researchers identified, from the more than 27,000 participants, 58 regions of interest within the genome in Caucasians, nine in African Americans, and 16 in Hispanics. These regions appeared independent of the well-known human leukocyte antigen (HLA) associations, also studied in depth here. An important observation was that nearly 50% of these regions had multiple genetic variants that predispose someone to SLE.
“These include 24 novel SLE regions…refined association signals in established regions, extended associations to additional ancestries, and a disentangled complex HLA multigenic effect,” the authors wrote. “The risk allele count (genetic load) exhibits an accelerating pattern of SLE risk, leading us to posit a cumulative hit hypothesis for autoimmune disease. Comparing results across the three ancestries identifies both ancestry-dependent and ancestry-independent contributions to SLE risk.”
Interestingly the investigators identified that as the number of genetic risk variants a person has increases, the risk for SLE increases more than expected if the variants were working independently. These observations led the authors to propose what they called a “cumulative hits hypothesis for autoimmune disease.”
Looking toward the future, the research team hopes to understand better how these genetic variants influence the risk of lupus, identify any possible drug targets, and determine if any environmental factors, such as infections, can trigger the development of the disease in someone who has a genetic susceptibility. They emphasize that it is important to increase the number of understudied populations, such as African American and Hispanic, to better understand the genetic causes of health disparities in lupus and the unique risks in all ethnic groups.
“We are delighted to see the work we funded on the ImmunoChip come to fruition and congratulate Dr. Langefeld along with his colleagues on this tremendous success,” said Kenneth Farber, J.D., CEO and president of the Lupus Research Alliance. “This study is among the few to concentrate heavily on non-Caucasian populations for a significantly broader evaluation while utilizing the most current and comprehensive information about human DNA.”